 |
 |

Strategies for Dealing With Amoxicillin Failure in Acute Otitis Media
Stan L. Block, MD
Arch Fam Med. 1999;8:68-78.
ABSTRACT
 |  |
Acute otitis media is the most common bacterial infection in pediatric patients. The predominant pathogens of acute otitis media are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Traditionally, amoxicillin has been the first-line therapeutic choice for patients with uncomplicated acute otitis media. However, with the increasing isolation of  -lactamase–producing organisms and penicillin-resistant S pneumoniae, the frequency of amoxicillin treatment failures also appears to be increasing. Several issues should be considered when alternative antibiotics are selected to treat amoxicillin failures, such as the most likely pathogens with their susceptibility patterns, and antibiotic issues including clinical efficacy for specific pathogens, adverse reactions, palatability, dosing schedules, and cost. Consequently, enhanced -lactamase–producing organisms and penicillin-resistant S pneumoniae, the frequency of amoxicillin treatment failures also appears to be increasing. Several issues should be considered when alternative antibiotics are selected to treat amoxicillin failures, such as the most likely pathogens with their susceptibility patterns, and antibiotic issues including clinical efficacy for specific pathogens, adverse reactions, palatability, dosing schedules, and cost. Consequently, enhanced  -lactamase stability, activity against penicillin-resistant S pneumoniae, and once- or twice-daily dosing regimens must be considered when antibiotics are chosen for patients in whom amoxicillin therapy has failed. -lactamase stability, activity against penicillin-resistant S pneumoniae, and once- or twice-daily dosing regimens must be considered when antibiotics are chosen for patients in whom amoxicillin therapy has failed.
INTRODUCTION
The perfect antibiotic for acute otitis media (AOM) has yet to be developed. Such an antibiotic would be active against the common gram-positive and gram-negative bacteria that cause AOM, including penicillin-susceptible and penicillin-resistant Streptococcus pneumoniae,  -lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, and group A Streptococcus. Unfortunately, many antibiotics that cover gram-positive organisms relinquish some coverage for gram-negative bacteria, and vice versa. Recently, the more frequent isolation of antibiotic-resistant bacteria has made management of AOM more problematic.1-2 Multidrug-resistant S pneumoniae are becoming more common in upper respiratory tract infections and in invasive diseases.3-6 The majority of strains of the gram-negative organisms, H influenzae and M catarrhalis, currently isolated from children with AOM produce -lactamase–producing Haemophilus influenzae and Moraxella catarrhalis, and group A Streptococcus. Unfortunately, many antibiotics that cover gram-positive organisms relinquish some coverage for gram-negative bacteria, and vice versa. Recently, the more frequent isolation of antibiotic-resistant bacteria has made management of AOM more problematic.1-2 Multidrug-resistant S pneumoniae are becoming more common in upper respiratory tract infections and in invasive diseases.3-6 The majority of strains of the gram-negative organisms, H influenzae and M catarrhalis, currently isolated from children with AOM produce  -lactamase enzymes, which often confer resistance to various -lactamase enzymes, which often confer resistance to various  -lactam antibiotics.1-2,7-8 -lactam antibiotics.1-2,7-8
Because accurate identification of infectious pathogens in AOM requires tympanocentesis (an invasive procedure almost never performed by clinicians), antibiotic selection for AOM is usually based on published reports, recommendations from respected authorities, and local hospital susceptibility patterns of pathogens normally obtained from adults with invasive disease. Unfortunately, the reported susceptibility patterns of these pathogens may not accurately reflect the often greater degree of resistance found in pediatric upper respiratory tract isolates.9-11 For instance, Doern and associates10-11 compared the patterns of resistance for strains of S pneumoniae and H influenzae obtained from middle ear fluid (MEF) in children and from sputum in adults. The rates of  -lactamase–producing H influenzae were 46% and 32%, respectively, and rates for penicillin-resistant S pneumoniae (PRSP) were 42% and 25%, respectively. -lactamase–producing H influenzae were 46% and 32%, respectively, and rates for penicillin-resistant S pneumoniae (PRSP) were 42% and 25%, respectively.
Amoxicillin is considered the first-line therapeutic choice for children with uncomplicated AOM because it is usually effective, inexpensive, and well tolerated.12 However, because amoxicillin lacks stability against  -lactamases and may not be effective against emerging multidrug-resistant strains of S pneumoniae, amoxicillin failures can be expected to occur more frequently in clinical practice.12-14 Therefore, when amoxicillin therapy fails, practitioners must often choose between antibiotics that possess higher activity in vitro against either PRSP or -lactamases and may not be effective against emerging multidrug-resistant strains of S pneumoniae, amoxicillin failures can be expected to occur more frequently in clinical practice.12-14 Therefore, when amoxicillin therapy fails, practitioners must often choose between antibiotics that possess higher activity in vitro against either PRSP or  -lactamase–producing organisms. Amoxicillin failures can be defined as cases in which (1) signs and symptoms of AOM persist or worsen after 48 hours of therapy with amoxicillin or (2) signs of AOM recur within 14 days after therapy, regardless of symptoms.15 Signs of AOM include discoloration such as redness (not pinkness); yellow, white (nonscarred), or green opacification; or purulent, cloudy air fluid levels, usually with decreased mobility.16 Otitis media with effusion is commonly observed in 50% to 60% of younger children after AOM.17 In otitis media with effusion, the tympanic membrane appears dull or orange, is not inflamed, and does not merit further antibiotic therapy. -lactamase–producing organisms. Amoxicillin failures can be defined as cases in which (1) signs and symptoms of AOM persist or worsen after 48 hours of therapy with amoxicillin or (2) signs of AOM recur within 14 days after therapy, regardless of symptoms.15 Signs of AOM include discoloration such as redness (not pinkness); yellow, white (nonscarred), or green opacification; or purulent, cloudy air fluid levels, usually with decreased mobility.16 Otitis media with effusion is commonly observed in 50% to 60% of younger children after AOM.17 In otitis media with effusion, the tympanic membrane appears dull or orange, is not inflamed, and does not merit further antibiotic therapy.
ACUTE OTITIS MEDIA
Acute otitis media is the most common bacterial illness requiring a visit to the clinician's office.18 In 1990, patients with AOM accounted for 24.5 million office visits12, 18 and spent approximately $240 million on antibiotics.19 Established risk factors for AOM are younger age; white, Alaskan Eskimo, or Native American heritage; family history of AOM; household members who smoke; and cleft palate.3, 12, 20 Child care attendance is considered a major risk factor for AOM because of its inherent crowding and because it leads to markedly increased recurrent infections and antibiotic exposures, with further selective pressure for resistant strains of bacteria.21 Subsequently, children in child care centers have a 7-fold higher incidence of tympanostomy tube placement than children who do not attend child care (21% vs 3%, respectively).22
Some practitioners have advocated the withholding of antibiotics initially for AOM, a highly controversial option exercised in some European countries. This premise is based on spontaneous cure rates of 14% to 88% observed with placebo therapy and the worsening bacterial resistance rates occurring throughout the United States.23 However, the humane considerations of treating AOM with relatively safe and benign drugs must be considered in pediatric patients, particularly those who cannot verbalize their complaints.24 Allowing patients to unnecessarily suffer for several days with pain, sleeplessness, irritability, and fever may not be an acceptable alternative to most clinicians (who would likewise be the first to treat themselves or their own crying child with AOM), parents (who anguish over their child's pain), and managed care organizations (who regularly survey for patient satisfaction). Potential suppurative complications, such as bacteremia, mastoiditis, chronic purulent otorrhea, and permanent hearing loss, also may occur.12, 15, 23, 25-26 Consequently, most experts believe that children with clinically diagnosed AOM should receive empirical antibiotic therapy because of the beneficial therapeutic-toxicity ratio.15, 27
On the other hand, overtreating viral and nonbacterial illnesses with antibiotics is never an acceptable alternative. First, practitioners must be accurate in their diagnosis of AOM by using a proper halogen-illuminated pneumatic otoscope, the largest appropriately sized 3.5-cm (1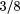 -in) speculum to fit the auditory canal (ie, dispose of all "disposable" speculums because of their truncated length and too small or too large sizing), and careful, thorough cleansing of the auditory canal of cerumen when necessary. The tympanic membrane must be critically visualized. Second, telephone history, parental history, or the child's complaints of otalgia should not be relied on as the sole basis for the diagnosis of AOM because of their frequent lack of correlation with actual physical findings. Probably the largest single factor fostering antibiotic resistance is the careless misuse and overuse of antibiotics for misdiagnosed viral or bacterial infections and for antimicrobial prophylaxis. -in) speculum to fit the auditory canal (ie, dispose of all "disposable" speculums because of their truncated length and too small or too large sizing), and careful, thorough cleansing of the auditory canal of cerumen when necessary. The tympanic membrane must be critically visualized. Second, telephone history, parental history, or the child's complaints of otalgia should not be relied on as the sole basis for the diagnosis of AOM because of their frequent lack of correlation with actual physical findings. Probably the largest single factor fostering antibiotic resistance is the careless misuse and overuse of antibiotics for misdiagnosed viral or bacterial infections and for antimicrobial prophylaxis.
COMMON PATHOGENS IN AOM
Acute otitis media is a bacterial disease in most cases. Although viral infections frequently precipitate AOM, viruses usually exist as copathogens with bacteria and are isolated as the sole pathogen in merely 6% of cases of AOM.24, 28-29 High rates (>85%) of bacterial pathogen recovery after tympanocentesis have been observed with prompt plating on standard media and incubating of aspirates in the office. Initial inoculation of the aspirate onto transport media should be avoided.3 Among patients with previously untreated AOM, pneumococci are recovered in 30% to 50%, H influenzae in 20% to 30%, and M catarrhalis in 5% to 20% (Figure 1).3, 5, 12, 30 Block and colleagues3 also found that H influenzae and M catarrhalis occurred as copathogens in 10% of children with penicillin-susceptible S pneumoniae and in 25% of those with PRSP.3, 13, 23
Recently, Block and colleagues31 isolated Chlamydia pneumoniae in 8% of 101 children with AOM undergoing tympanocentesis. Yet it was the sole pathogen in only 2 of these 8 children. Contrary to conventional wisdom that atypical pathogens infect only older patients, all of these patients were younger than 60 months, and 5 of these 8 children were younger than 16 months. In contrast, among a population of young children undergoing insertion of pressure equalization tubes in Seattle, Wash, Goo and associates32 were not able to isolate C pneumoniae from children with chronic otitis media. On the other hand, Mycoplasma pneumoniae is rarely, if ever, isolated from the MEF of children with AOM.33 In vitro, both of these atypical pathogens are resistant to  -lactam and sulfa antibiotics and susceptible to macrolides, tetracyclines, and quinolones. However, tetracycline and quinolones should not be used in children younger than 8 years and 15 years, respectively. -lactam and sulfa antibiotics and susceptible to macrolides, tetracyclines, and quinolones. However, tetracycline and quinolones should not be used in children younger than 8 years and 15 years, respectively.
BACTERIAL RESISTANCE IN AOM
Among aerobic bacteria isolated from the middle ear, bacterial resistance to amoxicillin is mediated predominantly by 2 mechanisms: production of  -lactamase enzymes (H influenzae and M catarrhalis) and modification of penicillin-binding proteins (S pneumoniae). Before the 1990s, most strains of S pneumoniae were susceptible to penicillin, with a minimum inhibitory concentration (MIC) of 0.06 µg/mL or less. However, in this decade, penicillin-resistant pneumococci (intermediate, 0.1-1.0 µg/mL; resistant, -lactamase enzymes (H influenzae and M catarrhalis) and modification of penicillin-binding proteins (S pneumoniae). Before the 1990s, most strains of S pneumoniae were susceptible to penicillin, with a minimum inhibitory concentration (MIC) of 0.06 µg/mL or less. However, in this decade, penicillin-resistant pneumococci (intermediate, 0.1-1.0 µg/mL; resistant, 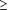 2.0 µg/mL) have emerged as endemic pathogens throughout the United States, with isolation rates of 40% from the nasopharynx of children in child care centers.34-35 Block and colleagues3 first reported PRSP as a common causative pathogen (17%) of AOM from healthy children. They identified significant risk factors for isolation of PRSP from children with AOM: attendance in a child care center, refractory AOM, the otitis-prone condition, and age younger than 24 months. The PRSP are particularly encountered in patients who have refractory AOM (44%) vs patients who have nonrefractory AOM (9%) (Figure 2). Risk factors for recovering PRSP from children with systemic disease or nasopharyngeal carriage include younger age, recent hospitalization, and, foremost, previous 2.0 µg/mL) have emerged as endemic pathogens throughout the United States, with isolation rates of 40% from the nasopharynx of children in child care centers.34-35 Block and colleagues3 first reported PRSP as a common causative pathogen (17%) of AOM from healthy children. They identified significant risk factors for isolation of PRSP from children with AOM: attendance in a child care center, refractory AOM, the otitis-prone condition, and age younger than 24 months. The PRSP are particularly encountered in patients who have refractory AOM (44%) vs patients who have nonrefractory AOM (9%) (Figure 2). Risk factors for recovering PRSP from children with systemic disease or nasopharyngeal carriage include younger age, recent hospitalization, and, foremost, previous  -lactam exposure.4, 35-36 -lactam exposure.4, 35-36
Streptococcus pneumoniae has developed resistance to  -lactam antibiotics by altering its penicillin-binding proteins, which decreases its binding affinity for these antibiotics.1 Furthermore, these pneumococci often simultaneously acquire degradation enzymes that bestow resistance to sulfonamides, tetracyclines, chloramphenicol, and macrolide antibiotics. Practitioners should note that, although the term penicillin resistance is used to describe S pneumoniae, the level of in vitro resistance to other antibiotics is commonly higher than it is to amoxicillin (Table 1).43-44 Currently, all strains of PRSP are susceptible to vancomycin, and most are susceptible to imipenem, meropenem, clindamycin, and rifampin. -lactam antibiotics by altering its penicillin-binding proteins, which decreases its binding affinity for these antibiotics.1 Furthermore, these pneumococci often simultaneously acquire degradation enzymes that bestow resistance to sulfonamides, tetracyclines, chloramphenicol, and macrolide antibiotics. Practitioners should note that, although the term penicillin resistance is used to describe S pneumoniae, the level of in vitro resistance to other antibiotics is commonly higher than it is to amoxicillin (Table 1).43-44 Currently, all strains of PRSP are susceptible to vancomycin, and most are susceptible to imipenem, meropenem, clindamycin, and rifampin.
|
|

|
|
Table 1. The Likelihood of an Antibiotic MEF Concentration Exceeding MIC50 or MIC90 Values for a Particular Pathogen in Pediatric Patients With AOM*
|
|
|
In the past, after amoxicillin failure,  -lactamase–producing H influenzae and M catarrhalis appeared to be the most common isolates.13 Nontypable H influenzae, which is not included in the H influenzae type B vaccine, accounts for 20% to 30% of isolates recovered by tympanocentesis (Figure 1).3, 42 In the 1980s, approximately 35% of H influenzae strains produced -lactamase–producing H influenzae and M catarrhalis appeared to be the most common isolates.13 Nontypable H influenzae, which is not included in the H influenzae type B vaccine, accounts for 20% to 30% of isolates recovered by tympanocentesis (Figure 1).3, 42 In the 1980s, approximately 35% of H influenzae strains produced  -lactamase and were resistant to ampicillin.1-2,45-46 However, the incidence of -lactamase and were resistant to ampicillin.1-2,45-46 However, the incidence of  -lactamase–producing H influenzae has increased to 55% in recent years.16, 47 These -lactamase–producing H influenzae has increased to 55% in recent years.16, 47 These  -lactamase–producing organisms confer resistance to amoxicillin and reduced susceptibility to most second-generation cephalosporins, and even to trimethoprim-sulfamethoxazole (Table 1). Many strains of H influenzae, regardless of -lactamase–producing organisms confer resistance to amoxicillin and reduced susceptibility to most second-generation cephalosporins, and even to trimethoprim-sulfamethoxazole (Table 1). Many strains of H influenzae, regardless of  -lactamase production, possess reduced susceptibility to erythromycin and sometimes to clarithromycin. -lactamase production, possess reduced susceptibility to erythromycin and sometimes to clarithromycin.
Moraxella catarrhalis accounts for 5% to 20% of AOM isolates (Figure 1).46 Currently, nearly all strains of M catarrhalis produce  -lactamase and are resistant to amoxicillin.1-2,23 However, the -lactamase and are resistant to amoxicillin.1-2,23 However, the  -lactamase enzymes produced by M catarrhalis are comparatively weaker than those of H influenzae, and multiple-antibiotic resistance is uncommon.1-2 -lactamase enzymes produced by M catarrhalis are comparatively weaker than those of H influenzae, and multiple-antibiotic resistance is uncommon.1-2
Group A Streptococcus, an uncommon cause of AOM in younger children, accounts for about 13% of cases in children older than 48 months.47 Group A Streptococcus should be suspected in older children with concomitant AOM and pharyngitis, particularly during a streptococcal epidemic. Although penicillin resistance has never been reported with this organism, penicillin and erythromycin failures have been noted in 20% to 30% of patients with streptococcal pharyngitis.48-51 Although persistence of streptococcal pharyngitis in some patients may result from a carrier state, we observed that most children with bacteriological recurrences had growth of more than 10 colony-forming units per plate and also had recurrence of milder signs and symptoms of streptococcal pharyngitis. Furthermore, streptococci persisted in only 1 of 22 patients treated with a second course of antibiotics.49
A NEW ALGORITHM FOR ANTIBIOTIC SELECTION
In the past, clinicians have relied on MIC break points alone to determine in vitro susceptibility and subsequent antibiotic choices. Unfortunately, susceptibility break points for S pneumoniae have not been established for most oral cephalosporins (except cefuroxime) by the National Committee for Clinical Laboratory Standards. Furthermore, susceptibility break points tend to vary from year to year and do not account for the notable differences in achievable concentration of antibiotic at the site of infection, particularly in MEF. Thus, another algorithm to predict antibiotic efficacy for AOM is needed.
In vivo clinical cures for AOM may be more reliably predicted when the peak antibiotic concentration in the MEF exceeds the in vitro bacterial MIC for a reasonable period.52-53 For instance, achievable MEF concentrations vary considerably between antibiotics depending on dosing, half-life, prodrug status (eg, cefuroxime axetil, cefpodoxime proxetil), gastrointestinal tract absorption, tissue penetration, and timing of the measurement. Accuracy of MEF concentrations also may be confounded by the type of assay performed, contamination by blood, and whether the specimen was obtained from children with AOM or otitis media with effusion. Despite these caveats, the MEF concentrations of certain antibiotics can probably be grouped. Antibiotics with relatively higher MEF concentrations include the new macrolide-azalides (ie, clarithromycin, azithromycin), new cephalosporins (ie, ceftibuten, loracarbef), and high-dose amoxicillin (Table 2).52, 54, 56, 60 Although the MEF concentration of the sulfonamide component of combination sulfonamide antibiotics is also relatively high, the MEF fluid concentration obtained with the trimethoprim or erythromycin component is notably low (approximately 0.5 µg/mL).39 The MEF concentrations of all other antibiotics approved for AOM range from about 0.8 µg/mL to 2.0 µg/mL.38-39,55, 57
|
|

|
|
Table 2. Oral Antibiotics With High MEF Penetration in Acute Otitis Media*
|
|
|
SELECTING ALTERNATIVE ANTIMICROBIAL AGENTS AFTER AMOXICILLIN FAILURE
As initial therapy, younger children who tend to have AOM caused by PRSP may respond better to higher doses (60-80 mg/kg per day divided twice or 3 times daily) of amoxicillin.3, 58-60 Higher doses of amoxicillin increase achievable peak MEF concentration from 3 to approximately 6 µg/mL, which is well above the MIC of most intermediately PRSP.58, 60-61 Nonetheless, amoxicillin should not be used as a first-line agent in children who are allergic to penicillin, in whom 2 documented treatment courses during the otitis media season have failed, or who have experienced a recurrence within 2 weeks after therapy with amoxicillin.23 However, trimethoprim-sulfamethoxazole, cefixime, and ceftibuten may be poor alternative choices for first-line therapy in these patients because of their suboptimal overall pneumococcal coverage.
Using the ratio of serum concentrations to bacterial MICs as an algorithm for predicting AOM efficacy, Craig and Andes44 calculated that amoxicillin and amoxicillin plus clavulanate potassium are the only oral  -lactam antibiotics with serum concentrations that exceed the MIC of intermediate PRSP for an acceptable interval after oral administration of standard doses. This may be important for the potentially bacteremic patient.44 However, serum concentrations usually moderately overestimate MEF concentrations of most antibiotics, with the exception of the 2 new macrolides (azithromycin and clarithromycin), whose MEF concentrations greatly exceed their serum concentrations. In addition, only amoxicillin plus clavulanate (3-times-daily formulation), cefprozil, and cefuroxime have demonstrated some in vivo efficacy against PRSP in a limited number of patients.3, 8, 16, 62 -lactam antibiotics with serum concentrations that exceed the MIC of intermediate PRSP for an acceptable interval after oral administration of standard doses. This may be important for the potentially bacteremic patient.44 However, serum concentrations usually moderately overestimate MEF concentrations of most antibiotics, with the exception of the 2 new macrolides (azithromycin and clarithromycin), whose MEF concentrations greatly exceed their serum concentrations. In addition, only amoxicillin plus clavulanate (3-times-daily formulation), cefprozil, and cefuroxime have demonstrated some in vivo efficacy against PRSP in a limited number of patients.3, 8, 16, 62
Unfortunately, no large series of good comparative data exist to evaluate the in vivo efficacy of second-line antibiotics in patients for whom these antibiotics are actually targeted (eg, antibiotic failures). Also, data are sparse regarding the causative pathogens in patients with refractory AOM, frequently a different group epidemiologically. Data from Block and colleagues3 have shown that PRSP may be recovered in up to 44% of these patients (Figure 2). Thus, practitioners may encounter much higher failure rates in second- and third-line treatment, possibly as high as 50% to 60% depending on the drug, among recently treated patients who have been antibiotic failures. This contradicts the high success rates reported in clinical trials, which nearly always select only patients with nonrefractory AOM.
After amoxicillin failure, antibiotic therapy for at least 10 to 14 days should be directed against either  -lactamase–producing organisms, such as M catarrhalis and H influenzae, or PRSP.13-14,23 Previously, -lactamase–producing organisms, such as M catarrhalis and H influenzae, or PRSP.13-14,23 Previously,  -lactamase–producing H influenzae and M catarrhalis have been the pathogens presumed to cause the majority of clinical failures in amoxicillin-treated patients.23 Earlier data from Brook and Yocum13 suggested that H influenzae may be the most common pathogen associated with amoxicillin failure. -lactamase–producing H influenzae and M catarrhalis have been the pathogens presumed to cause the majority of clinical failures in amoxicillin-treated patients.23 Earlier data from Brook and Yocum13 suggested that H influenzae may be the most common pathogen associated with amoxicillin failure.
In the past, standard second-line therapy for AOM has included trimethoprim-sulfamethoxazole, cefaclor, and erythromycin-sulfisoxazole. Although these agents have been reasonable second-line choices, decreasing susceptibility to both PRSP and H influenzae and potential adverse effects may limit their usefulness in clinical practice. The generic forms of the 2 sulfonamide antibiotics tend to be less palatable. Trimethoprim-sulfamethoxazole is reported as one of the more common oral antibiotics that cause Stevens-Johnson syndrome, an extremely rare, potentially fatal hypersensitivity reaction.63 Because erythromycin-sulfisoxazole must be administered 4 times daily, compliance is markedly hindered. It also causes gastritis in up to 15% of patients, may cost more than azithromycin for a child weighing 15 kg or more, and uses 2 antibiotics, which could increase resistance. Cefaclor has been associated with serum sickness–like reaction, characterized by erythema multiforme, arthralgia, and fever, in up to 1.5% of children.63 In addition, recent in vitro data comparing these 3 agents have shown increased resistance among both  -lactamase–producing gram-negative organisms and PRSP (Table 1).64 In general, most of the newer antibiotics, including third-generation cephalosporins, macrolide-azalide antibiotics, and amoxicillin-clavulanate (twice daily), produce lower rates of moderate to severe adverse effects, significantly improve dosing and tissue penetration pharmacokinetics, and enhance in vitro coverage when compared with older agents. Consequently, in my opinion only the newer antibiotics and possibly trimethoprim-sulfamethoxazole should be considered for patients with AOM that is unresponsive to amoxicillin or other antibiotics. -lactamase–producing gram-negative organisms and PRSP (Table 1).64 In general, most of the newer antibiotics, including third-generation cephalosporins, macrolide-azalide antibiotics, and amoxicillin-clavulanate (twice daily), produce lower rates of moderate to severe adverse effects, significantly improve dosing and tissue penetration pharmacokinetics, and enhance in vitro coverage when compared with older agents. Consequently, in my opinion only the newer antibiotics and possibly trimethoprim-sulfamethoxazole should be considered for patients with AOM that is unresponsive to amoxicillin or other antibiotics.
Second-Generation Cephalosporins
Cefuroxime, cefprozil, and loracarbef are commonly used second-line antibiotics for patients with AOM.59 All 3 antibiotics are dosed twice daily. Cefuroxime is more active in vivo than in vitro against PRSP and has activity against  -lactamase–producing strains of H influenzae and M catarrhalis.30, 62 However, poor palatability limits its utility in the liquid formulation, with the exception of younger infants who are still bottle feeding, where the antibiotic can be mixed into the liquid. A new, possibly more palatable formulation of the 250-mg suspension has become available. Cefprozil possesses reasonable gram-positive coverage against Staphylococcus aureus, group A Streptococcus, and intermediately PRSP.30, 65 However, it is only moderately active against H influenzae and is hydrolyzed by -lactamase–producing strains of H influenzae and M catarrhalis.30, 62 However, poor palatability limits its utility in the liquid formulation, with the exception of younger infants who are still bottle feeding, where the antibiotic can be mixed into the liquid. A new, possibly more palatable formulation of the 250-mg suspension has become available. Cefprozil possesses reasonable gram-positive coverage against Staphylococcus aureus, group A Streptococcus, and intermediately PRSP.30, 65 However, it is only moderately active against H influenzae and is hydrolyzed by  -lactamase enzymes.66 Loracarbef is a carbacephem antibiotic that is similar to cefaclor, but has a somewhat improved spectrum of activity for H influenzae and M catarrhalis.30 Unlike cefaclor, loracarbef has better pharmacokinetics for twice-daily dosing and is not associated with serum sickness. All 3 of these second-generation cephalosporins are less active than third-generation cephalosporins against H influenzae and M catarrhalis (Table 1). -lactamase enzymes.66 Loracarbef is a carbacephem antibiotic that is similar to cefaclor, but has a somewhat improved spectrum of activity for H influenzae and M catarrhalis.30 Unlike cefaclor, loracarbef has better pharmacokinetics for twice-daily dosing and is not associated with serum sickness. All 3 of these second-generation cephalosporins are less active than third-generation cephalosporins against H influenzae and M catarrhalis (Table 1).
Third-Generation Cephalosporins
The third-generation oral cephalosporins, ceftibuten dihydrate, cefixime, cefpodoxime proxetil, and the newest cephalosporin, cefdinir, are reasonable alternatives for the treatment of AOM in patients in whom amoxicillin therapy has failed, particularly in areas with low rates of PRSP.67-70 Ceftibuten and cefixime are dosed once daily; cefdinir and cefpodoxime may be dosed once or twice daily.30 Among the oral cephalosporins, ceftibuten has the highest activity against H influenzae.71 Cefpodoxime and possibly cefdinir appear to have the broadest overall spectrum among the oral cephalosporins, with much better coverage of penicillin-susceptible S pneumoniae and intermediate PRSP (Table 1). 42, 68, 70 However, in vitro data suggest that twice-daily dosing of cefpodoxime provides better coverage of H influenzae compared with once-daily dosing.72
The MIC50 and MIC90 are the concentrations of antibiotic that inhibit 50% and 90% of bacterial growth, respectively. All 4 oral third-generation cephalosporins are extremely active against  -lactamase–producing strains of H influenzae and M catarrhalis,57, 68, 73 but ceftibuten is least active against M catarrhalis (MIC90, 4.0 µg/mL).74 It is also the least active against penicillin-susceptible S pneumoniae (MIC90, 4.0-8.0 µg/mL) when compared with cefixime (MIC90, 0.5 µg/mL) and cefpodoxime (MIC90, <0.06 µg/mL). However, because ceftibuten achieves the highest MEF concentrations (at 4 hours, mean of 4.0 µg/mL in 6 patients and 14.3 µg/mL in 2 patients) when compared with cefixime (mean of 1.32 µg/mL) and cefpodoxime (mean of 0.87 µg/mL), the MEF concentrations may exceed the MICs for many of these organisms (Table 2).38, 41, 44, 56, 75-76 Neither cefixime nor ceftibuten possesses much in vitro activity against PRSP, whereas cefpodoxime exceeds the MIC50 values of intermediate PRSP.74, 77 Cefdinir is currently being studied. -lactamase–producing strains of H influenzae and M catarrhalis,57, 68, 73 but ceftibuten is least active against M catarrhalis (MIC90, 4.0 µg/mL).74 It is also the least active against penicillin-susceptible S pneumoniae (MIC90, 4.0-8.0 µg/mL) when compared with cefixime (MIC90, 0.5 µg/mL) and cefpodoxime (MIC90, <0.06 µg/mL). However, because ceftibuten achieves the highest MEF concentrations (at 4 hours, mean of 4.0 µg/mL in 6 patients and 14.3 µg/mL in 2 patients) when compared with cefixime (mean of 1.32 µg/mL) and cefpodoxime (mean of 0.87 µg/mL), the MEF concentrations may exceed the MICs for many of these organisms (Table 2).38, 41, 44, 56, 75-76 Neither cefixime nor ceftibuten possesses much in vitro activity against PRSP, whereas cefpodoxime exceeds the MIC50 values of intermediate PRSP.74, 77 Cefdinir is currently being studied.
Recently approved as a single-dose therapy for AOM, ceftriaxone also provides excellent coverage against the major pathogens associated with AOM.77 Nonetheless, clinicians must be aware that MIC90 values of ceftriaxone sodium for PRSP have risen as high as 4.0 µg/mL and that MEF concentrations dropped to 0.74 µg/mL by 24 hours in one earlier study.3 These observations raise concerns about the use of single-dose ceftriaxone in 2 groups of children whose AOM is commonly associated with PRSP: those younger than 24 months and those with refractory AOM (Figure 2).47 Single-dose ceftriaxone has demonstrated efficacy equivalent merely to that of trimethoprim-sulfamethoxazole in a clinical trial that studied children with presumptive evidence of AOM (eg, middle ear effusion and symptoms of acute illness). However, specific signs of AOM (discoloration or purulent effusion) were not necessary for enrollment, tympanocentesis was not performed (thus the study included mild and moderate AOM), and clinical cures were determined by lack of symptoms only and not by lack of tympanic membrane inflammation. Thus, single-dose ceftriaxone should be reserved only for moderate to severe cases of previously untreated AOM in patients who are (1) poorly compliant, (2) unable to take oral medications because of gastroenteritis, or (3) moderately ill but still treatable as outpatients (who should then be followed up and prescribed an active pneumococcal oral agent for 7 to 10 days). When ceftriaxone is selected to treat children with AOM suspected or identified as having PRSP, duration of therapy should be 3 to 5 days.3, 78 Its widespread indiscriminate use may hasten resistance to this invaluable drug in pediatrics.
Macrolide-Azalide Antibiotics
Other reasonable antibiotic alternatives for AOM after amoxicillin failure include the new macrolide-azalide antibiotics, clarithromycin and azithromycin. Both agents provide markedly enhanced MEF concentrations, tissue and leukocyte penetration, and reduced gastrointestinal tract adverse effects compared with erythromycin.79 Strains of S pneumoniae that are resistant to erythromycin have typically been considered resistant to clarithromycin and azithromycin as well.80 Nonetheless, when compared with erythromycin, clarithromycin and azithromycin achieve notably higher MEF concentrations that subsequently exceed the MIC90 values for intermediate PRSP and the MIC50 values for PRSP (Table 1). Although the in vitro data suggest a role for the newer macrolides in the treatment of AOM caused by PRSP, in vivo efficacy against these resistant strains is unknown.
Clarithromycin achieves high MEF concentrations, particularly when its active 14-hydroxy metabolite is included. The MEF concentrations of clarithromycin and 14-hydroxyclarithromycin 12 hours after the sixth oral dose were 7.4 µg/mL and 3.8 µg/mL, respectively (Table 2).52 Although clarithromycin displays modest in vitro activity against penicillin-resistant strains of S pneumoniae, it has relatively high MICs for H influenzae (8-16 µg/mL) (Table 1). In adults, the active 14-hydroxy metabolite of clarithromycin may provide both an additive and a synergistic effect in vitro against H influenzae, probably because adults have acquired antibody to nontypable H influenzae from previous infections.81 This phenomenon has not been reported in children. Clarithromycin also has enhanced gram-positive activity when compared with erythromycin.79
Azithromycin achieves prolonged high MEF concentrations (8.6 µg/mL at 24 hours and 9.4 µg/mL at 48 hours), and a 5-day course of therapy has demonstrated in vivo efficacy equivalent to a 10-day course of therapy with standard oral antibiotics, such as amoxicillin-clavulanate (Table 2).40 Azithromycin is more active against H influenzae and slightly less active against S pneumoniae compared with clarithromycin (Table 1). A 5-day course of therapy with once-daily administration, as well as its palatability, enhances patient compliance.79
Amoxicillin-Clavulanate
Amoxicillin-clavulanate is a good alternative for the treatment of children in whom amoxicillin therapy has failed, with the exception of those with penicillin hypersensitivity or concomitant gastroenteritis. It possesses a broad spectrum of in vitro activity, achieves reasonable MEF concentrations, and has demonstrated in vivo efficacy in clinical trials that have included patients with severe AOM requiring tympanocentesis or those identified with PRSP.3, 16 In fact, in the 1990s, the US Food and Drug Administration has uniformly chosen it as the drug against which all new antibiotics in clinical trials studying children undergoing tympanocentesis for severe AOM are compared. Although serious adverse effects are rarely reported with the older 3-times-daily formulation, diarrhea has been a relatively common adverse effect, particularly in younger children. However, the new twice-daily formulation (amoxicillin with clavulanate, 45:6.4 mg/kg per day) reduces the incidence of diarrhea by half, improves compliance over the old formulation, and is more palatable.82 Antibiotic-induced diarrhea also may be reduced by preceding the administration of this drug with food.83-84
OTHER THERAPEUTIC ISSUES AFTER AMOXICILLIN FAILURE
In general, oral antibiotics used to treat AOM have a low incidence of severe adverse reactions. The most common side effects are gastrointestinal tract disturbances, hypersensitivity reactions, and rashes (particularly diaper dermatitis). The newer antimicrobial agents or formulations tend to have a lower incidence of gastrointestinal tract side effects, most of which are mild to moderate. The reported frequency of diarrhea for some newer antibiotics is as follows: cefixime, 16%; amoxicillin-clavulanate twice daily, 9%; cefpodoxime proxetil, 7%; azithromycin, 5%; and ceftibuten, 4%.42, 83, 85-86
The taste of liquid formulations is critical for compliance in children.12, 30 In a study comparing the smell, body, taste, and aftertaste of 15 pediatric antibiotic liquid formulations, ceftibuten, cefaclor, and cefadroxil were the most palatable suspensions.87 Cephalosporin suspensions seemed to have a more pleasant taste and were better accepted by patients when compared with suspensions that contained penicillin.88 Antibiotics with particular palatability problems include cefuroxime and clarithromycin. Anecdotally, the palatability of clarithromycin is markedly improved when liquid "chasers" (eg, fruit punch) are administered before and after dosing.
Because frequent dosing regimens decrease patient compliance,89 compliance rates in children improve with antibiotics that are dosed once or twice daily.23, 30, 90 Antibiotics with approved once-daily dosing include the third-generation cephalosporins and azithromycin. All other oral antibiotics for AOM, except erythromycin-sulfisoxazole, may be dosed twice daily.91 However, some evidence suggests that a twice-daily cefaclor regimen may be less effective than a 3-times-daily regimen.92
Cost is an important issue for patients in the private sector and in managed care programs. Nonetheless, the cost-effectiveness of antibiotic therapy must be correlated with a variety of factors, including efficacy, patient compliance, the incidence of adverse reactions, drug interactions, and treatment failures.19, 79, 93 Antibiotics associated with low rates of failure and recurrence should be considered the most cost-effective therapy for the management of AOM.94
MANAGEMENT OF AOM IN THE 1990s
It is important to remember that once initial antibiotic therapy has failed in a child, the rate of either spontaneous or antibiotic-mediated resolution is markedly diminished. Furthermore, selecting an antibiotic after amoxicillin failure may be critically linked to the prevalence of PRSP in the local community. Data from the local community (usually adult data) or children's hospital may be representative of the prevalence. However, data from the respiratory tracts of ambulatory children would be more reliable. Clinicians can better assess local prevalence of PRSP by culturing the following sites in ambulatory children younger than 36 months during the otitis season (October through May): spontaneously draining pressure equalization tubes, nasopharynx of young children in child care centers, and purulent conjunctivitis.35, 95-96 The approach to antibiotic selection is dependent on an estimate of the prevalence of PRSP in the local community (Figure 3).
|
|

|
|
Figure 3. Algorithm for treating acute otitis media (AOM) in the 1990s. BID indicates twice daily; PRSP, penicillin-resistant Streptococcus pneumoniae; and TID, 3 times daily.3, 9, 78
|
|
|
In areas with low rates (<20%) of PRSP,  -lactamase–producing organisms may be more common after amoxicillin failure (Figure 3). Thus, -lactamase–producing organisms may be more common after amoxicillin failure (Figure 3). Thus,  -lactam and non– -lactam and non– -lactam antibiotics with better gram-negative coverage would be preferred. If second-line therapy with a -lactam antibiotics with better gram-negative coverage would be preferred. If second-line therapy with a  -lactam antibiotic fails, then theoretically a non– -lactam antibiotic fails, then theoretically a non– -lactam antibiotic should be chosen for third-line therapy because of its different mechanism of action. The converse would also be true. In areas with high rates ( -lactam antibiotic should be chosen for third-line therapy because of its different mechanism of action. The converse would also be true. In areas with high rates (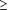 20%) of PRSP, antibiotics with better pneumococcal coverage should be selected as either second- or third-line therapy (Figure 3). As described earlier, the same premise would apply regarding choosing a 20%) of PRSP, antibiotics with better pneumococcal coverage should be selected as either second- or third-line therapy (Figure 3). As described earlier, the same premise would apply regarding choosing a  -lactam antibiotic for patients in whom therapy with a non– -lactam antibiotic for patients in whom therapy with a non– -lactam antibiotic has failed, and vice versa. -lactam antibiotic has failed, and vice versa.
In either case, for fourth-line therapy, PRSP should be suspected and antibiotics specifically targeting these pathogens should be selected: oral clindamycin hydrochloride, 20 mg/kg per day divided 3 times daily; ceftriaxone, 50 mg/kg per day for a minimum of 3 to 5 days; or, if not used yet, combination amoxicillin–clavulanate (45 mg/kg per day) plus amoxicillin (40 mg/kg per day), simultaneously administered twice daily.3, 78, 97 However, clindamycin does not cover H influenzae, has poor palatability, and rarely causes pseudomembranous colitis. Adding the clavulanate component to high-dose amoxicillin provides coverage for  -lactamase–producing gram-negative organisms, rather than enhancing activity against PRSP. Subsequent treatment failure with any of these regimens would require prompt otolaryngological referral for placement of ventilating tubes. -lactamase–producing gram-negative organisms, rather than enhancing activity against PRSP. Subsequent treatment failure with any of these regimens would require prompt otolaryngological referral for placement of ventilating tubes.
CASE ANALYSIS
When the clinician is confronted with amoxicillin failure in a patient with AOM, concomitant signs and symptoms, such as pneumonia, conjunctivitis, vomiting, and diarrhea, should help narrow particular antibiotic choices, as shown in the following examples.
Case 1
A 3-year-old in whom amoxicillin therapy for AOM failed has now developed concomitant afebrile pneumonia.
The atypical pathogens, M pneumoniae and C pneumoniae, may account for up to one half of cases of community-acquired pneumonia in ambulatory children aged 3 through 12 years.98 Although M pneumoniae is not a pathogen in AOM,34 C pneumoniae may be a pathogen in up to 8% of patients with AOM, regardless of the presence or absence of lower respiratory tract infection.31  -Lactam and sulfonamide antibiotics provide minimal in vitro coverage against these atypical pathogens. Thus, clarithromycin or azithromycin would be the preferred choices for this child because they provide coverage both for the "atypical" pathogens of pneumonia and for the "typical" pathogens of AOM. Although erythromycin-sulfisoxazole may be used as an alternative, its marginal spectrum of coverage for AOM pathogens, inconvenient dosing regimen (ie, 4 times daily), erythromycin-mediated gastritis, and potential for sulfa reactions limit its usefulness. -Lactam and sulfonamide antibiotics provide minimal in vitro coverage against these atypical pathogens. Thus, clarithromycin or azithromycin would be the preferred choices for this child because they provide coverage both for the "atypical" pathogens of pneumonia and for the "typical" pathogens of AOM. Although erythromycin-sulfisoxazole may be used as an alternative, its marginal spectrum of coverage for AOM pathogens, inconvenient dosing regimen (ie, 4 times daily), erythromycin-mediated gastritis, and potential for sulfa reactions limit its usefulness.
Case 2
A moderately ill and appropriately immunized 15-month-old with persistent AOM and clear lungs has subsequently developed high fever and moderate leukocytosis while receiving amoxicillin.
Because the H influenzae type B vaccine has virtually eliminated infections caused by H influenzae type B,99 clinicians should be most concerned about infection with S pneumoniae in this patient. In light of worsening clinical symptoms despite amoxicillin therapy, which presumably eradicates penicillin-susceptible pneumococci, PRSP would be the most likely pathogen.38 Hospitalization may be necessary. For outpatient therapy, because of the distinct possibility of occult bacteremia, blood cultures should be obtained, and parenteral ceftriaxone (50 mg/kg per day) for 3 to 5 days may be preferred in this case, depending on severity of the illness and concomitant gastrointestinal tract symptoms. In areas with documented low rates of clindamycin resistance, clindamycin (20-30 mg/kg per day 3 times daily) could be administered for 7 to 10 days, either orally (as long as the child is observed for retention of the antibiotic) or parenterally, at least initially.44, 99 Despite the reported high success rate of treatment with  -lactam antibiotics for PRSP, either therapeutic choice merits vigilant follow-up during the next 24 to 48 hours.21, 100-101 For outpatient therapy, trustworthy caretakers and careful follow-up within the first 24 hours is critical. Any patient who subsequently does not respond or worsens requires a chest radiograph and hospitalization. -lactam antibiotics for PRSP, either therapeutic choice merits vigilant follow-up during the next 24 to 48 hours.21, 100-101 For outpatient therapy, trustworthy caretakers and careful follow-up within the first 24 hours is critical. Any patient who subsequently does not respond or worsens requires a chest radiograph and hospitalization.
Case 3
A 10-month-old child in whom amoxicillin therapy for the treatment of AOM failed has now developed an abrupt onset of bilateral purulent conjunctivitis.
Concurrent AOM is observed in up to two thirds of children with purulent conjunctivitis.102-103 The pathogen most frequently associated with conjunctivitis-otitis syndrome is H influenzae, and nearly one half of these strains currently produce  -lactamase. Both susceptible and resistant strains of S pneumoniae are occasionally isolated (S.L.B., unpublished data, September 1998). If Gram stain or culture from the discharge of the eye is not available or if no organisms or pleomorphic gram-negative rods are recovered, amoxicillin-clavulanate or a third-generation cephalosporin, such as ceftibuten, cefixime, cefdinir, or cefpodoxime, or possibly azithromycin, should be selected. First-generation cephalosporins, such as cephalexin, should not be used for AOM because of their minimal MEF penetration and lack of H influenzae coverage. -lactamase. Both susceptible and resistant strains of S pneumoniae are occasionally isolated (S.L.B., unpublished data, September 1998). If Gram stain or culture from the discharge of the eye is not available or if no organisms or pleomorphic gram-negative rods are recovered, amoxicillin-clavulanate or a third-generation cephalosporin, such as ceftibuten, cefixime, cefdinir, or cefpodoxime, or possibly azithromycin, should be selected. First-generation cephalosporins, such as cephalexin, should not be used for AOM because of their minimal MEF penetration and lack of H influenzae coverage.
Case 4
An 18-month-old boy with AOM who has completed 2 days of his amoxicillin therapy has now developed lymphadenitis and impetigo of the nose.
Staphylococcus aureus and occasionally group A Streptococcus are the pathogens most commonly implicated in impetigo and lymphadenitis. Although S aureus is rarely a causative pathogen in AOM, it is cultured in more than 95% of patients with impetigo and bacterial lymphadenitis. Furthermore, group A Streptococcus, the other pathogen of impetigo, should have responded to amoxicillin. Thus, this patient is infected with 2 distinct bacterial pathogens: S aureus and pathogens of AOM. Because 20% of S aureus isolates are resistant to macrolide-azalide antibiotics and increasing resistance to cefaclor has been reported, these agents should be used only with reservation in this patient. In addition, third-generation cephalosporins possess minimal or unreliable S aureus coverage and should not be used. Cefpodoxime is approved for the treatment of S aureus only in adults using double the standard dose.83
Thus, the preferred antibiotic for this patient would be either amoxicillin-clavulanate or a second-generation cephalosporin.
Case 5
Clinical examination of a 30-month-old child who has developed persistent vomiting and diarrhea after receiving amoxicillin for 9 days reveals bulging, erythematous tympanic membranes.
Antibiotics commonly associated with some degree of gastrointestinal tract distress, such as amoxicillin-clavulanate, erythromycin-sulfisoxazole, and clarithromycin, should not be used in this patient. Antibiotics that require liquid "chasers" to prevent aftertaste (eg, clarithromycin, cefuroxime, and cefpodoxime) should also be avoided. Appropriate choices for this patient include the more palatable  -lactamase–stable antibiotics which produce minimal gastrointestinal tract adverse effects, such as ceftibuten, cefixime, azithromycin, and possibly trimethoprim-sulfamethoxazole. If clinicians suspect the possibility of bacterial enteritis (high fever or blood or leukocytes in the stool), then azithromycin should be avoided. -lactamase–stable antibiotics which produce minimal gastrointestinal tract adverse effects, such as ceftibuten, cefixime, azithromycin, and possibly trimethoprim-sulfamethoxazole. If clinicians suspect the possibility of bacterial enteritis (high fever or blood or leukocytes in the stool), then azithromycin should be avoided.
CONCLUSIONS
The primary pathogens that cause AOM after amoxicillin failure include  -lactamase–producing gram-negative organisms (ie, H influenzae and M catarrhalis) and PRSP. Choosing antibiotics for children with AOM in whom amoxicillin therapy failed is a complex decision and should be based on careful consideration of the most likely pathogens, data on rates of PRSP from local ambulatory populations, and ancillary factors, such as concomitant infections including gastroenteritis, pneumonia, impetigo, conjunctivitis, or potential bacteremia. Practitioners often must choose between antibiotics with enhanced -lactamase–producing gram-negative organisms (ie, H influenzae and M catarrhalis) and PRSP. Choosing antibiotics for children with AOM in whom amoxicillin therapy failed is a complex decision and should be based on careful consideration of the most likely pathogens, data on rates of PRSP from local ambulatory populations, and ancillary factors, such as concomitant infections including gastroenteritis, pneumonia, impetigo, conjunctivitis, or potential bacteremia. Practitioners often must choose between antibiotics with enhanced  -lactamase stability or antibiotics with potential activity against PRSP. Adverse reactions, product taste, costs, dosing regimens, and compliance issues also may have a role in determining the most effective and well-accepted agent for the treatment of AOM after amoxicillin failure. -lactamase stability or antibiotics with potential activity against PRSP. Adverse reactions, product taste, costs, dosing regimens, and compliance issues also may have a role in determining the most effective and well-accepted agent for the treatment of AOM after amoxicillin failure.
AUTHOR INFORMATION
Accepted for publication January 26, 1998.
Corresponding author: Stan L. Block, MD, Kentucky Pediatric Research, 201 S Fifth St, Bardstown, KY 40004.
From Kentucky Pediatric Research, Bardstown.
REFERENCES
 |  |
1. Doern GV. Resistance among problem respiratory pathogens in pediatrics. Pediatr Infect Dis J. 1995;14:420-423.
ISI
| PUBMED
2. Doern GV. Trends in antimicrobial susceptibility of bacterial pathogens of the respiratory tract. Am J Med. 1995;99(suppl 6B):3S-7S.
3. Block SL, Harrison CJ, Hedrick JA, et al. Penicillin-resistant Streptococcus pneumoniae in acute otitis media: risk factors, susceptibility patterns, and antimicrobial management. Pediatr Infect Dis J. 1995;14:751-759.
ISI
| PUBMED
4. Hofmann J, Cetron MS, Farley MM. The prevalence of drug-resistant Streptococcus pneumoniae in Atlanta. N Engl J Med. 1995;333:481-486.
FREE FULL TEXT
5. Mason EO, Kaplan SL. Penicillin-resistant pneumococci in the United States. Pediatr Infect Dis J. 1995;14:1017-1018.
FULL TEXT
|
ISI
| PUBMED
6. McDougal LK, Rasheed JK, Biddle JW, et al. Identification of multiple clones of extended-spectrum cephalosporin-resistant Streptococcus pneumoniae isolates in the United States. Antimicrob Agents Chemother. 1995;39:2282-2288.
ABSTRACT
7. McLinn S, Williams D. Incidence of antibiotic-resistant Streptococcus pneumoniae and  -lactamase–positive Haemophilus influenzae in clinical isolates from patients with otitis media. Pediatr Infect Dis J. 1996;15(suppl 9):S3-S9.
8. Pichichero ME. Empiric antibiotic selection criteria for respiratory infections in pediatric practice. Pediatr Infect Dis J. 1997;16(suppl 3):S60-S64.
9. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
FULL TEXT
|
ISI
| PUBMED
10. Doern GV, Brueggemann A, Holley HP Jr, Rauch AM. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208-1213.
ABSTRACT
11. Doern GV, Brueggemann AB, Pierce G, Hogan T, Holley HP Jr, Rauch A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:2884-2886.
ABSTRACT
12. Klein JO. Otitis media. Clin Infect Dis. 1994;19:823-833.
ISI
| PUBMED
13. Brook I, Yocum P. Bacteriology and -lactamase–positive Haemophilus influenzae in clinical isolates from patients with otitis media. Pediatr Infect Dis J. 1996;15(suppl 9):S3-S9.
8. Pichichero ME. Empiric antibiotic selection criteria for respiratory infections in pediatric practice. Pediatr Infect Dis J. 1997;16(suppl 3):S60-S64.
9. Block SL. Causative pathogens, antibiotic resistance and therapeutic considerations in acute otitis media. Pediatr Infect Dis J. 1997;16:449-456.
FULL TEXT
|
ISI
| PUBMED
10. Doern GV, Brueggemann A, Holley HP Jr, Rauch AM. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208-1213.
ABSTRACT
11. Doern GV, Brueggemann AB, Pierce G, Hogan T, Holley HP Jr, Rauch A. Prevalence of antimicrobial resistance among 723 outpatient clinical isolates of Moraxella catarrhalis in the United States in 1994 and 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:2884-2886.
ABSTRACT
12. Klein JO. Otitis media. Clin Infect Dis. 1994;19:823-833.
ISI
| PUBMED
13. Brook I, Yocum P. Bacteriology and  -lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr Infect Dis J. 1995;14:805-807.
ISI
| PUBMED
14. Pichichero ME, Pichichero CL. Persistent acute otitis media, II: antimicrobial treatment. Pediatr Infect Dis J. 1995;14:183-188.
ISI
| PUBMED
15. Paradise JL. Managing otitis media: a time for change [commentary]. Pediatrics. 1995;96:712-715.
FREE FULL TEXT
16. Hoberman A, Paradise JL, Block S, Burch DJ, Jacobs MR, Balanescu MI. Efficacy of amoxicillin/clavulanate for acute otitis media: relation to Streptococcus pneumoniae susceptibility. Pediatr Infect Dis J. 1996;15(suppl):955-962.
17. Klein JO. Selection of oral antimicrobial agents for otitis media and pharyngitis. Infect Dis Clin Pract. 1995;4(suppl 2):S88-S94.
18. Stool SE, Berg AO, Berman S, et al. Otitis Media With Effusion in Young Children. Rockville, Md: Agency for Health Care Policy and Research; 1994. AHCPR publication 94-0622.
19. Oh PI, Maerov P, Pritchard D, Knowles SR, Einarson TR, Shear NH. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Ther. 1996;18:160-182.
FULL TEXT
|
ISI
| PUBMED
20. Klein JO. Current issues in upper respiratory tract infections in infants and children: rationale for antibacterial therapy. Pediatr Infect Dis J. 1994;13:S5-S8.
21. Friedland IR, McCracken GH. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377-382.
FREE FULL TEXT
22. Wald ER, Dashefsky B, Byers C, Guerra N, Taylor F. Frequency and severity of infections in day care. J Pediatr. 1988;112:540-546.
FULL TEXT
|
ISI
| PUBMED
23. Pichichero ME. Assessing the treatment alternatives for acute otitis media. Pediatr Infect Dis J. 1994;13:S27-S34.
24. Howie VM. Otitis media. Pediatr Rev. 1993;14:320-323.
FREE FULL TEXT
25. Paradise JL. Short-course antimicrobial treatment for acute otitis media: not best for infants and young children. JAMA. 1997;278:1640-1642.
FREE FULL TEXT
26. Berman S. Otitis media in developing countries. Pediatrics. 1995;96:126-131.
FREE FULL TEXT
27. Rosenfeld RM, Vertrees JE, Carr J, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: meta-analysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994;124:355-367.
FULL TEXT
|
ISI
| PUBMED
28. Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120:856-862.
FULL TEXT
|
ISI
| PUBMED
29. Sung BS, Chonmaitree T, Broemeling LD, et al. Association of rhinovirus infection with poor bacteriologic outcome of bacterial-viral otitis media. Clin Infect Dis. 1993;17:38-42.
ISI
| PUBMED
30. Schatz BS, Karavokiros KT, Taeubel MA, Itokazu GS. Comparison of cefprozil, cefpodoxime proxetil, loracarbef, cefixime, and ceftibuten. Ann Pharmacother. 1996;30:258-268.
ABSTRACT
31. Block S, Hammerschlag MR, Hedrick J, et al. Chlamydia pneumoniae in acute otitis media. Pediatr Infect Dis J. 1997;16:858-862.
FULL TEXT
|
ISI
| PUBMED
32. Goo YA, Hori MK, Voorhies JH, Kou CC, Wang SP, Campbell LA. Failure to detect Chlamydia pneumoniae in ear fluids from children with otitis media. Pediatr Infect Dis J. 1995;14:1000-1001.
FULL TEXT
|
ISI
| PUBMED
33. Klein JO, Teele DW. Isolations of viruses and mycoplasmas from middle ear effusions: a review. Ann Otol Rhinol Laryngol. 1976;85(suppl 25):140-144.
34. Boken DJ, Chartrand SA, Goering RV, et al. Colonization with penicillin-resistant Streptococcus pneumoniae in a child-care center. Pediatr Infect Dis J. 1995;14:879-884.
ISI
| PUBMED
35. Duchin JS, Breiman RF, Diamond A, et al. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745-750.
ISI
| PUBMED
36. Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious use of antibiotics. Am Fam Physician. 1997;55:1647-1654.
ISI
| PUBMED
37. Fraschini F, Braga PC, Scarpazza G, et al. Human pharmacokinetics and distribution in various tissues of ceftriaxone. Chemotherapy. 1986;32:192-199.
ISI
| PUBMED
38. Harrison CJ, Chartrand SA, Rodriguez W, et al. Middle ear effusion concentrations of cefixime during acute otitis media with effusion and otitis media with effusion. Pediatr Infect Dis J. 1997;16:816-817.
FULL TEXT
|
ISI
| PUBMED
39. Nelson CT, Mason EO, Kaplan SL. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585-589.
ISI
| PUBMED
40. Powers JL. Properties of azithromycin that enhance the potential for compliance in children with upper respiratory tract infections. Pediatr Infect Dis J. 1996;15(suppl):S30-S37.
41. van Dyk J, Terespolsky SA, Meyer CS, van Niekerk CH, Klugman KP. Penetration of cefpodoxime into middle ear fluid in pediatric patients with acute otitis media. Pediatr Infect Dis J. 1997;16:79-81.
FULL TEXT
|
ISI
| PUBMED
42. Mendelman PM, Del Baccaro MA, McLinn SE, Todd WM. Cefpodoxime proxetil compared with amoxicillin-clavulanate for the treatment of otitis media. J Pediatr. 1992;121:459-465.
FULL TEXT
|
ISI
| PUBMED
43. Appelbaum PC. Epidemiology and in vitro susceptibility of drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1996;15:932-934.
FULL TEXT
|
ISI
| PUBMED
44. Craig WA, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255-259.
FULL TEXT
|
ISI
| PUBMED
45. McLinn SE, Moskal M, Goldfarb J, et al. Comparison of cefuroxime axetil and amoxicillin-clavulanate suspensions in treatment of acute otitis media with effusion in children. Antimicrob Agents Chemother. 1994;38:315-318.
FREE FULL TEXT
46. Pichichero ME, Pichichero CL. Persistent acute otitis media, I: causative pathogens. Pediatr Infect Dis J. 1995;14:178-183.
ISI
| PUBMED
47. Block S, Hedrick J, Harrison CJ, Chartrand S. Pathogens of acute otitis media in a pediatric population. Presented at: 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 16, 1996; New Orleans, La.
48. Bass JW. Antibiotic management of group A streptococcal pharyngotonsillitis. Pediatr Infect Dis J. 1991;10(suppl):S43-S49.
49. Block SL, Hedrick JA, Tyler RD. Comparative study of the effectiveness of cefixime and penicillin V for the treatment of Streptococcus pharyngitis in children and adolescents. Pediatr Infect Dis J. 1992;11:919-925.
ISI
| PUBMED
50. Coonan KM, Kaplan EL. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr Infect Dis J. 1994;13:630-635.
ISI
| PUBMED
51. Hsueh PR, Chen HM, Huang AH, Wu JJ. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239-2242.
ABSTRACT
52. Gan VN, McCarty JM, Chu SY, Carr R. Penetration of clarithromycin into middle ear fluid of children with acute otitis media. Pediatr Infect Dis J. 1997;16:39-43.
FULL TEXT
|
ISI
| PUBMED
53. Harrison CJ. Using antibiotic concentrations in middle ear fluid to predict potential clinical efficacy. Pediatr Infect Dis J. 1997;16(suppl):S12-S16.
54. Krause PJ, Owens NJ, Nightingale CH, et al. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serious otitis media. J Infect Dis. 1982;145:815-821.
ISI
| PUBMED
55. Kuszmiez H, Shelton S, Brown O, Manning S, Nelson JD. Loracarbef concentrations in middle ear fluid. Antimicrob Agents Chemother. 1990;34:2030-2031.
FREE FULL TEXT
56. Lin C, Kumari P, Perrotta RJ, Reidenberg BE. Penetration of ceftibuten into middle ear fluid. Antimicrob Agents Chemother. 1996;40:1394-1396.
ABSTRACT
57. Neu HC. Otitis media: antibiotic resistance of causative pathogens and treatment alternatives. Pediatr Infect Dis J. 1995;14(suppl):S51-S56.
58. Lister PD, Pong A, Chartrand SA, Sanders CC. Rationale behind high-dose amoxicillin therapy for acute otitis media due to penicillin-nonsusceptible pneumococci: support from in vitro pharmacodynamic studies. Antimicrob Agents Chemother. 1997;41:1926-1932.
ABSTRACT
59. Bradley JS, Kaplan SL, Klugman KP, Leggiardro RJ. Consensus: management of infections in children caused by Streptococcus pneumoniae with decreased susceptibility to penicillin. Pediatr Infect Dis J. 1995;14:1037-1041.
ISI
| PUBMED
60. Seikel K, Shelton S, McCracken GH Jr. Middle ear fluid concentrations of amoxicillin after large dosages in children with acute otitis media. Pediatr Infect Dis J. 1997;16:710-711.
FULL TEXT
|
ISI
| PUBMED
61. Friedland IR. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr Infect Dis J. 1995;14:885-890.
ISI
| PUBMED
62. Barry B, Gehanno P, Blumen M, Boucot I. Clinical outcome of acute otitis media caused by pneumococci with decreased susceptibility to penicillin. Scand J Infect Dis. 1994;26:446-452.
ISI
| PUBMED
63. Platt R, Dreis MW, Kennedy DL, et al. Serum sickness–like reactions to amoxicillin, cefaclor, cephalexin, and trimethoprim-sulfamethoxazole. J Infect Dis. 1988;158:474-477.
ISI
| PUBMED
64. Block S, Hedrick J, Harrison CJ, Chartrand S. MICs of H influenzae and M catarrhalis from acute otitis media. Presented at: 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 18, 1995; San Francisco, Calif.
65. Wiseman LR, Benfield P. Cefprozil: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1993;45:295-317.
ISI
| PUBMED
66. Barriere SL. Review of the in vitro activity, pharmacokinetic characteristics, safety, and clinical efficacy of cefprozil, a new oral cephalosporin. Ann Pharmacother. 1993;27:1082-1089.
ABSTRACT
67. Blumer JL, McLinn SE, DeAbate CA, et al. Multinational, multicenter controlled trial comparing ceftibuten with cefaclor for the treatment of acute otitis media. Pediatr Infect Dis J. 1995;14(suppl):S115-S120.
68. Frampton JE, Brogden RN, Langtry HD, Buckley MM. Cefpodoxime proxetil: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1992;44:889-917.
ISI
| PUBMED
69. McLinn SE, McCarty JM, Perrotta R, et al. Multicenter controlled trial comparing ceftibuten with amoxicillin/clavulanate in the empiric treatment of acute otitis media. Pediatr Infect Dis J. 1995;14:S108-S114.
70. McCarty JM, Hedrick JA, Block SL, Keyserling CH, Tack KJ and the Cefdinir Otitis Media Study Group. Comparative safety and efficacy of cefdinir vs amoxicillin/clavulanate for treatment of acute suppurative otitis media in children. Pediatr Infect Dis J. In press.
71. Abramowicz M. Ceftibuten: a new oral cephalosporin. Med Lett Drugs Ther. 1996;38:23-24.
PUBMED
72. Garrison MW, Malone CL, Eiland JE. Activity of once-daily cefpodoxime regimens against Haemophilus influenzae and Streptococcus pneumoniae with an in vitro pharmacodynamic chamber model. Antimicrob Agents Chemother. 1996;40:1545-1547.
ABSTRACT
73. Brogden RN, Campoli-Richards DM. Cefixime: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1989;38:524-550.
ISI
| PUBMED
74. Doern GV. Does there exist a rational, objective in vitro basis for the management of selected infections for the respiratory tract? Infect Dis Clin Pract. 1995;42(suppl 2):S68-S73.
75. Barr WH, Affrime M, Lin CC, Batra V. Pharmacokinetics of ceftibuten in children. Pediatr Infect Dis J. 1995;14(suppl):S93-S101.
76. Spector S. Review of the properties and features of ceftibuten: a new orally active antibiotic. Infect Dis Clin Pract. 1995;4(suppl 2):S113-S123.
77. Pankuch GA, Visalli MA, Jacobs MR, Appelbaum PC. Activities of oral and parenteral agents against penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1995;39:1499-1504.
ABSTRACT
78. Peter G. 1997 Red Book: Report of the Committee on Infectious Disease. 24th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1997:416.
79. Guay DR. Macrolide antibiotics in paediatric infectious disease. Drugs. 1996;51:515-536.
ISI
| PUBMED
80. Neu HC. Clinical microbiology of azithromycin. Am J Med. 1991;91(suppl 3A):12S-18S.
81. Peters DH, Clissold SP. Clarithromycin: a review of its antimicrobial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1992;44:117-164.
ISI
| PUBMED
82. Hoberman A, Paradise JL, Burch DJ, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin®) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463-470.
FULL TEXT
|
ISI
| PUBMED
83. Physicians' Desk Reference. 51st ed. Montvale, NJ: Medical Economics Data Production Co; 1998.
84. Block S, Hedrick J, Sperling M, McCarty J. A comparison of two different doses of Augmentin in the treatment of otitis media. Presented at: 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy; October 18, 1993; New Orleans, La.
85. Hopkins S, Williams D. Clinical tolerability and safety of azithromycin in children. Pediatr Infect Dis J. 1995;14(suppl):S67-S71.
86. Wiseman LR, Balfour JA. Ceftibuten: a review of its antibacterial activity, pharmacokinetic properties, and clinical efficacy. Drugs. 1994;47:784-808.
ISI
| PUBMED
87. Ortiz BG, Simental PS, Porras MH, et al. Comparison of the smell, body, taste, and aftertaste produced by 15 oral pediatric antibiotic liquid formulations. Presented at: 19th International Congress of Chemotherapy; July 16, 1995; Montreal, Quebec.
88. Ruff ME, Schotik DA, Bass JW, Vincent JM. Antimicrobial drug suspensions: a blind comparison of taste of fourteen common pediatric drugs. Pediatr Infect Dis J. 1991;10:30-33.
ISI
| PUBMED
89. Davey P, Parker S. Cost-effectiveness of once-daily oral antimicrobial therapy. J Clin Pharmacol. 1992;32:706-710.
ABSTRACT
90. Wandstrat TL, Kaplan B. Pharmacoeconomic impact of factors affecting compliance with antibiotic regimens in the treatment of acute otitis media. Pediatr Infect Dis J. 1997;16(suppl):S27-S29.
91. Hoberman A, Paradise JL, Burch DJ, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin®) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463-470.
92. Haines ST, Kraynak MA. Twice-daily cefaclor therapy. Ann Pharmacother. 1994;28:1353-1354.
ISI
| PUBMED
93. Berman S, Roark R, Luckey D. Theoretical cost-effectiveness of management options for children with persisting middle ear effusions. Pediatrics. 1994;93:353-363.
FREE FULL TEXT
94. Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. Pediatr Infect Dis J. 1997;16(suppl):S9-S11.
95. Block SL, Hedrick JA, Smith RA, et al. Culture of spontaneously draining acute otitis media (AOM): a practical epidemiologic technique for assessing community-wide resistance in pediatric AOM. Presented at: 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 29, 1997; Toronto, Ontario.
96. Boken DJ, Chartrand SA, Moland ES, Goering RV. Colonization with penicillin-nonsusceptible Streptococcus pneumoniae in urban and rural child-care centers. Pediatr Infect Dis J. 1996;15:667-672.
FULL TEXT
|
ISI
| PUBMED
97. Bottenfield GW, Burch DJ, Hedrick JA, Schaten R, Rowinski CA, Davies JT. Safety and tolerability of a new formulation (90 mg/kg/day divided every 12 h) of amoxicillin/clavulanate (Augmentin®) in the empiric treatment of pediatric acute otitis media caused by drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1998;17:963-968.
FULL TEXT
|
ISI
| PUBMED
98. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
ISI
| PUBMED
99. Givner LB, Woods CR, Abramson TR. The practice of pediatrics in the era of vaccines effective against Haemophilus influenzae type b. Pediatrics. 1994;93:680-681.
FREE FULL TEXT
100. Friedland AR, Istre GR. Management of penicillin-resistant pneumococcal infections. Pediatr Infect Dis J. 1992;11:433-435.
ISI
| PUBMED
101. Tan TQ, Mason EO, Kaplan SL. Systemic infections due to Streptococcus pneumoniae relatively resistant to penicillin in a children's hospital: clinical management and outcome. Pediatrics. 1992;90:928-933.
FREE FULL TEXT
102. Bodor FF. Conjunctivitis-otitis syndrome. Pediatrics. 1982;69:695-698.
FREE FULL TEXT
103. Harrison CJ, Hedrick JA, Block SL, Gilchrist MJ. Relation of the outcome of conjunctivitis and the conjunctivitis-otitis syndrome to identifiable risk factors and oral antimicrobial therapy. Pediatr Infect Dis J. 1987;6:536-540.
ISI
| PUBMED -lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr Infect Dis J. 1995;14:805-807.
ISI
| PUBMED
14. Pichichero ME, Pichichero CL. Persistent acute otitis media, II: antimicrobial treatment. Pediatr Infect Dis J. 1995;14:183-188.
ISI
| PUBMED
15. Paradise JL. Managing otitis media: a time for change [commentary]. Pediatrics. 1995;96:712-715.
FREE FULL TEXT
16. Hoberman A, Paradise JL, Block S, Burch DJ, Jacobs MR, Balanescu MI. Efficacy of amoxicillin/clavulanate for acute otitis media: relation to Streptococcus pneumoniae susceptibility. Pediatr Infect Dis J. 1996;15(suppl):955-962.
17. Klein JO. Selection of oral antimicrobial agents for otitis media and pharyngitis. Infect Dis Clin Pract. 1995;4(suppl 2):S88-S94.
18. Stool SE, Berg AO, Berman S, et al. Otitis Media With Effusion in Young Children. Rockville, Md: Agency for Health Care Policy and Research; 1994. AHCPR publication 94-0622.
19. Oh PI, Maerov P, Pritchard D, Knowles SR, Einarson TR, Shear NH. A cost-utility analysis of second-line antibiotics in the treatment of acute otitis media in children. Clin Ther. 1996;18:160-182.
FULL TEXT
|
ISI
| PUBMED
20. Klein JO. Current issues in upper respiratory tract infections in infants and children: rationale for antibacterial therapy. Pediatr Infect Dis J. 1994;13:S5-S8.
21. Friedland IR, McCracken GH. Management of infections caused by antibiotic-resistant Streptococcus pneumoniae. N Engl J Med. 1994;331:377-382.
FREE FULL TEXT
22. Wald ER, Dashefsky B, Byers C, Guerra N, Taylor F. Frequency and severity of infections in day care. J Pediatr. 1988;112:540-546.
FULL TEXT
|
ISI
| PUBMED
23. Pichichero ME. Assessing the treatment alternatives for acute otitis media. Pediatr Infect Dis J. 1994;13:S27-S34.
24. Howie VM. Otitis media. Pediatr Rev. 1993;14:320-323.
FREE FULL TEXT
25. Paradise JL. Short-course antimicrobial treatment for acute otitis media: not best for infants and young children. JAMA. 1997;278:1640-1642.
FREE FULL TEXT
26. Berman S. Otitis media in developing countries. Pediatrics. 1995;96:126-131.
FREE FULL TEXT
27. Rosenfeld RM, Vertrees JE, Carr J, et al. Clinical efficacy of antimicrobial drugs for acute otitis media: meta-analysis of 5400 children from thirty-three randomized trials. J Pediatr. 1994;124:355-367.
FULL TEXT
|
ISI
| PUBMED
28. Chonmaitree T, Owen MJ, Patel JA, Hedgpeth D, Horlick D, Howie VM. Effect of viral respiratory tract infection on outcome of acute otitis media. J Pediatr. 1992;120:856-862.
FULL TEXT
|
ISI
| PUBMED
29. Sung BS, Chonmaitree T, Broemeling LD, et al. Association of rhinovirus infection with poor bacteriologic outcome of bacterial-viral otitis media. Clin Infect Dis. 1993;17:38-42.
ISI
| PUBMED
30. Schatz BS, Karavokiros KT, Taeubel MA, Itokazu GS. Comparison of cefprozil, cefpodoxime proxetil, loracarbef, cefixime, and ceftibuten. Ann Pharmacother. 1996;30:258-268.
ABSTRACT
31. Block S, Hammerschlag MR, Hedrick J, et al. Chlamydia pneumoniae in acute otitis media. Pediatr Infect Dis J. 1997;16:858-862.
FULL TEXT
|
ISI
| PUBMED
32. Goo YA, Hori MK, Voorhies JH, Kou CC, Wang SP, Campbell LA. Failure to detect Chlamydia pneumoniae in ear fluids from children with otitis media. Pediatr Infect Dis J. 1995;14:1000-1001.
FULL TEXT
|
ISI
| PUBMED
33. Klein JO, Teele DW. Isolations of viruses and mycoplasmas from middle ear effusions: a review. Ann Otol Rhinol Laryngol. 1976;85(suppl 25):140-144.
34. Boken DJ, Chartrand SA, Goering RV, et al. Colonization with penicillin-resistant Streptococcus pneumoniae in a child-care center. Pediatr Infect Dis J. 1995;14:879-884.
ISI
| PUBMED
35. Duchin JS, Breiman RF, Diamond A, et al. High prevalence of multidrug-resistant Streptococcus pneumoniae among children in a rural Kentucky community. Pediatr Infect Dis J. 1995;14:745-750.
ISI
| PUBMED
36. Dowell SF, Schwartz B. Resistant pneumococci: protecting patients through judicious use of antibiotics. Am Fam Physician. 1997;55:1647-1654.
ISI
| PUBMED
37. Fraschini F, Braga PC, Scarpazza G, et al. Human pharmacokinetics and distribution in various tissues of ceftriaxone. Chemotherapy. 1986;32:192-199.
ISI
| PUBMED
38. Harrison CJ, Chartrand SA, Rodriguez W, et al. Middle ear effusion concentrations of cefixime during acute otitis media with effusion and otitis media with effusion. Pediatr Infect Dis J. 1997;16:816-817.
FULL TEXT
|
ISI
| PUBMED
39. Nelson CT, Mason EO, Kaplan SL. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585-589.
ISI
| PUBMED
40. Powers JL. Properties of azithromycin that enhance the potential for compliance in children with upper respiratory tract infections. Pediatr Infect Dis J. 1996;15(suppl):S30-S37.
41. van Dyk J, Terespolsky SA, Meyer CS, van Niekerk CH, Klugman KP. Penetration of cefpodoxime into middle ear fluid in pediatric patients with acute otitis media. Pediatr Infect Dis J. 1997;16:79-81.
FULL TEXT
|
ISI
| PUBMED
42. Mendelman PM, Del Baccaro MA, McLinn SE, Todd WM. Cefpodoxime proxetil compared with amoxicillin-clavulanate for the treatment of otitis media. J Pediatr. 1992;121:459-465.
FULL TEXT
|
ISI
| PUBMED
43. Appelbaum PC. Epidemiology and in vitro susceptibility of drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1996;15:932-934.
FULL TEXT
|
ISI
| PUBMED
44. Craig WA, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255-259.
FULL TEXT
|
ISI
| PUBMED
45. McLinn SE, Moskal M, Goldfarb J, et al. Comparison of cefuroxime axetil and amoxicillin-clavulanate suspensions in treatment of acute otitis media with effusion in children. Antimicrob Agents Chemother. 1994;38:315-318.
FREE FULL TEXT
46. Pichichero ME, Pichichero CL. Persistent acute otitis media, I: causative pathogens. Pediatr Infect Dis J. 1995;14:178-183.
ISI
| PUBMED
47. Block S, Hedrick J, Harrison CJ, Chartrand S. Pathogens of acute otitis media in a pediatric population. Presented at: 36th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 16, 1996; New Orleans, La.
48. Bass JW. Antibiotic management of group A streptococcal pharyngotonsillitis. Pediatr Infect Dis J. 1991;10(suppl):S43-S49.
49. Block SL, Hedrick JA, Tyler RD. Comparative study of the effectiveness of cefixime and penicillin V for the treatment of Streptococcus pharyngitis in children and adolescents. Pediatr Infect Dis J. 1992;11:919-925.
ISI
| PUBMED
50. Coonan KM, Kaplan EL. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr Infect Dis J. 1994;13:630-635.
ISI
| PUBMED
51. Hsueh PR, Chen HM, Huang AH, Wu JJ. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239-2242.
ABSTRACT
52. Gan VN, McCarty JM, Chu SY, Carr R. Penetration of clarithromycin into middle ear fluid of children with acute otitis media. Pediatr Infect Dis J. 1997;16:39-43.
FULL TEXT
|
ISI
| PUBMED
53. Harrison CJ. Using antibiotic concentrations in middle ear fluid to predict potential clinical efficacy. Pediatr Infect Dis J. 1997;16(suppl):S12-S16.
54. Krause PJ, Owens NJ, Nightingale CH, et al. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serious otitis media. J Infect Dis. 1982;145:815-821.
ISI
| PUBMED
55. Kuszmiez H, Shelton S, Brown O, Manning S, Nelson JD. Loracarbef concentrations in middle ear fluid. Antimicrob Agents Chemother. 1990;34:2030-2031.
FREE FULL TEXT
56. Lin C, Kumari P, Perrotta RJ, Reidenberg BE. Penetration of ceftibuten into middle ear fluid. Antimicrob Agents Chemother. 1996;40:1394-1396.
ABSTRACT
57. Neu HC. Otitis media: antibiotic resistance of causative pathogens and treatment alternatives. Pediatr Infect Dis J. 1995;14(suppl):S51-S56.
58. Lister PD, Pong A, Chartrand SA, Sanders CC. Rationale behind high-dose amoxicillin therapy for acute otitis media due to penicillin-nonsusceptible pneumococci: support from in vitro pharmacodynamic studies. Antimicrob Agents Chemother. 1997;41:1926-1932.
ABSTRACT
59. Bradley JS, Kaplan SL, Klugman KP, Leggiardro RJ. Consensus: management of infections in children caused by Streptococcus pneumoniae with decreased susceptibility to penicillin. Pediatr Infect Dis J. 1995;14:1037-1041.
ISI
| PUBMED
60. Seikel K, Shelton S, McCracken GH Jr. Middle ear fluid concentrations of amoxicillin after large dosages in children with acute otitis media. Pediatr Infect Dis J. 1997;16:710-711.
FULL TEXT
|
ISI
| PUBMED
61. Friedland IR. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr Infect Dis J. 1995;14:885-890.
ISI
| PUBMED
62. Barry B, Gehanno P, Blumen M, Boucot I. Clinical outcome of acute otitis media caused by pneumococci with decreased susceptibility to penicillin. Scand J Infect Dis. 1994;26:446-452.
ISI
| PUBMED
63. Platt R, Dreis MW, Kennedy DL, et al. Serum sickness–like reactions to amoxicillin, cefaclor, cephalexin, and trimethoprim-sulfamethoxazole. J Infect Dis. 1988;158:474-477.
ISI
| PUBMED
64. Block S, Hedrick J, Harrison CJ, Chartrand S. MICs of H influenzae and M catarrhalis from acute otitis media. Presented at: 35th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 18, 1995; San Francisco, Calif.
65. Wiseman LR, Benfield P. Cefprozil: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1993;45:295-317.
ISI
| PUBMED
66. Barriere SL. Review of the in vitro activity, pharmacokinetic characteristics, safety, and clinical efficacy of cefprozil, a new oral cephalosporin. Ann Pharmacother. 1993;27:1082-1089.
ABSTRACT
67. Blumer JL, McLinn SE, DeAbate CA, et al. Multinational, multicenter controlled trial comparing ceftibuten with cefaclor for the treatment of acute otitis media. Pediatr Infect Dis J. 1995;14(suppl):S115-S120.
68. Frampton JE, Brogden RN, Langtry HD, Buckley MM. Cefpodoxime proxetil: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1992;44:889-917.
ISI
| PUBMED
69. McLinn SE, McCarty JM, Perrotta R, et al. Multicenter controlled trial comparing ceftibuten with amoxicillin/clavulanate in the empiric treatment of acute otitis media. Pediatr Infect Dis J. 1995;14:S108-S114.
70. McCarty JM, Hedrick JA, Block SL, Keyserling CH, Tack KJ and the Cefdinir Otitis Media Study Group. Comparative safety and efficacy of cefdinir vs amoxicillin/clavulanate for treatment of acute suppurative otitis media in children. Pediatr Infect Dis J. In press.
71. Abramowicz M. Ceftibuten: a new oral cephalosporin. Med Lett Drugs Ther. 1996;38:23-24.
PUBMED
72. Garrison MW, Malone CL, Eiland JE. Activity of once-daily cefpodoxime regimens against Haemophilus influenzae and Streptococcus pneumoniae with an in vitro pharmacodynamic chamber model. Antimicrob Agents Chemother. 1996;40:1545-1547.
ABSTRACT
73. Brogden RN, Campoli-Richards DM. Cefixime: a review of its antibacterial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1989;38:524-550.
ISI
| PUBMED
74. Doern GV. Does there exist a rational, objective in vitro basis for the management of selected infections for the respiratory tract? Infect Dis Clin Pract. 1995;42(suppl 2):S68-S73.
75. Barr WH, Affrime M, Lin CC, Batra V. Pharmacokinetics of ceftibuten in children. Pediatr Infect Dis J. 1995;14(suppl):S93-S101.
76. Spector S. Review of the properties and features of ceftibuten: a new orally active antibiotic. Infect Dis Clin Pract. 1995;4(suppl 2):S113-S123.
77. Pankuch GA, Visalli MA, Jacobs MR, Appelbaum PC. Activities of oral and parenteral agents against penicillin-susceptible and -resistant pneumococci. Antimicrob Agents Chemother. 1995;39:1499-1504.
ABSTRACT
78. Peter G. 1997 Red Book: Report of the Committee on Infectious Disease. 24th ed. Elk Grove Village, Ill: American Academy of Pediatrics; 1997:416.
79. Guay DR. Macrolide antibiotics in paediatric infectious disease. Drugs. 1996;51:515-536.
ISI
| PUBMED
80. Neu HC. Clinical microbiology of azithromycin. Am J Med. 1991;91(suppl 3A):12S-18S.
81. Peters DH, Clissold SP. Clarithromycin: a review of its antimicrobial activity, pharmacokinetic properties, and therapeutic potential. Drugs. 1992;44:117-164.
ISI
| PUBMED
82. Hoberman A, Paradise JL, Burch DJ, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin®) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463-470.
FULL TEXT
|
ISI
| PUBMED
83. Physicians' Desk Reference. 51st ed. Montvale, NJ: Medical Economics Data Production Co; 1998.
84. Block S, Hedrick J, Sperling M, McCarty J. A comparison of two different doses of Augmentin in the treatment of otitis media. Presented at: 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy; October 18, 1993; New Orleans, La.
85. Hopkins S, Williams D. Clinical tolerability and safety of azithromycin in children. Pediatr Infect Dis J. 1995;14(suppl):S67-S71.
86. Wiseman LR, Balfour JA. Ceftibuten: a review of its antibacterial activity, pharmacokinetic properties, and clinical efficacy. Drugs. 1994;47:784-808.
ISI
| PUBMED
87. Ortiz BG, Simental PS, Porras MH, et al. Comparison of the smell, body, taste, and aftertaste produced by 15 oral pediatric antibiotic liquid formulations. Presented at: 19th International Congress of Chemotherapy; July 16, 1995; Montreal, Quebec.
88. Ruff ME, Schotik DA, Bass JW, Vincent JM. Antimicrobial drug suspensions: a blind comparison of taste of fourteen common pediatric drugs. Pediatr Infect Dis J. 1991;10:30-33.
ISI
| PUBMED
89. Davey P, Parker S. Cost-effectiveness of once-daily oral antimicrobial therapy. J Clin Pharmacol. 1992;32:706-710.
ABSTRACT
90. Wandstrat TL, Kaplan B. Pharmacoeconomic impact of factors affecting compliance with antibiotic regimens in the treatment of acute otitis media. Pediatr Infect Dis J. 1997;16(suppl):S27-S29.
91. Hoberman A, Paradise JL, Burch DJ, et al. Equivalent efficacy and reduced occurrence of diarrhea from a new formulation of amoxicillin/clavulanate potassium (Augmentin®) for treatment of acute otitis media in children. Pediatr Infect Dis J. 1997;16:463-470.
92. Haines ST, Kraynak MA. Twice-daily cefaclor therapy. Ann Pharmacother. 1994;28:1353-1354.
ISI
| PUBMED
93. Berman S, Roark R, Luckey D. Theoretical cost-effectiveness of management options for children with persisting middle ear effusions. Pediatrics. 1994;93:353-363.
FREE FULL TEXT
94. Kaplan B, Wandstrat TL, Cunningham JR. Overall cost in the treatment of otitis media. Pediatr Infect Dis J. 1997;16(suppl):S9-S11.
95. Block SL, Hedrick JA, Smith RA, et al. Culture of spontaneously draining acute otitis media (AOM): a practical epidemiologic technique for assessing community-wide resistance in pediatric AOM. Presented at: 37th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 29, 1997; Toronto, Ontario.
96. Boken DJ, Chartrand SA, Moland ES, Goering RV. Colonization with penicillin-nonsusceptible Streptococcus pneumoniae in urban and rural child-care centers. Pediatr Infect Dis J. 1996;15:667-672.
FULL TEXT
|
ISI
| PUBMED
97. Bottenfield GW, Burch DJ, Hedrick JA, Schaten R, Rowinski CA, Davies JT. Safety and tolerability of a new formulation (90 mg/kg/day divided every 12 h) of amoxicillin/clavulanate (Augmentin®) in the empiric treatment of pediatric acute otitis media caused by drug-resistant Streptococcus pneumoniae. Pediatr Infect Dis J. 1998;17:963-968.
FULL TEXT
|
ISI
| PUBMED
98. Block S, Hedrick J, Hammerschlag MR, Cassell GH, Craft JC. Mycoplasma pneumoniae and Chlamydia pneumoniae in pediatric community-acquired pneumonia: comparative efficacy and safety of clarithromycin vs erythromycin ethylsuccinate. Pediatr Infect Dis J. 1995;14:471-477.
ISI
| PUBMED
99. Givner LB, Woods CR, Abramson TR. The practice of pediatrics in the era of vaccines effective against Haemophilus influenzae type b. Pediatrics. 1994;93:680-681.
FREE FULL TEXT
100. Friedland AR, Istre GR. Management of penicillin-resistant pneumococcal infections. Pediatr Infect Dis J. 1992;11:433-435.
ISI
| PUBMED
101. Tan TQ, Mason EO, Kaplan SL. Systemic infections due to Streptococcus pneumoniae relatively resistant to penicillin in a children's hospital: clinical management and outcome. Pediatrics. 1992;90:928-933.
FREE FULL TEXT
102. Bodor FF. Conjunctivitis-otitis syndrome. Pediatrics. 1982;69:695-698.
FREE FULL TEXT
103. Harrison CJ, Hedrick JA, Block SL, Gilchrist MJ. Relation of the outcome of conjunctivitis and the conjunctivitis-otitis syndrome to identifiable risk factors and oral antimicrobial therapy. Pediatr Infect Dis J. 1987;6:536-540.
ISI
| PUBMED
RELATED ARTICLES
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 1999;8(1):23-25.
FULL TEXT
Bacterial Resistance Due to Antimicrobial Drug Addiction Among Physicians: Time for a Cure!
Jon S. Abramson and Laurence B. Givner
Arch Fam Med. 1999;8(1):79-80.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Bacterial Resistance Due to Antimicrobial Drug Addiction Among Physicians: Time for a Cure!
Abramson and Givner
Arch Fam Med 1999;8:79-80.
FULL TEXT
|