 |
 |

Clinical and Demographic Predictors of Late-Stage Cervical Cancer
Jeanne M. Ferrante, MD;
Eduardo C. Gonzalez, MD;
Richard G. Roetzheim, MD, MSPH;
Naazneen Pal, BA;
Laurie Woodard, MD
Arch Fam Med. 2000;9:439-445.
ABSTRACT
 |  |
Background Despite increasingly widespread use of the Papanicolaou smear, almost half of all women with invasive cervical cancer are diagnosed at a late stage (regional or distant). Little is known about factors associated with late-stage diagnosis of cervical cancer.
Objective To examine the relationship of age, race, education level, income level, smoking, marital status, health insurance type, comorbidity, and residence in an urban or rural setting to late stage at diagnosis of cervical cancer.
Methods Incident cases of invasive cervical cancer occurring in 1994 in Florida were identified from the state tumor registry (N=852). Cases were linked with state discharge abstracts and the 1990 US census. Multiple logistic regression was used to determine the relationship between predictor variables (age, race or ethnicity, marital status, smoking status, education level, income level, insurance type, comorbidity, and urban vs rural residence) and the odds of late-stage diagnosis.
Results Age, marital status, and insurance type were associated with late-stage diagnosis. Each additional year of age was associated with a 3% increased odds of late-stage diagnosis (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.02-1.05; P<.001). Being unmarried was associated with a 63% increased odds of late-stage diagnosis (OR, 1.63; 95% CI, 1.18-2.25; P=.003). Being uninsured was associated with a 60% increased odds of late-stage diagnosis (OR, 1.60; 95% CI, 1.07-2.38; P=.02). Having commercial health maintenance organization insurance was associated with a 46% decreased odds of late-stage disease (OR, 0.54; 95% CI, 0.30-0.96; P=.04). Race, education level, income level, smoking status, comorbidity, and urban residence were not associated with stage at diagnosis.
Conclusions Women with cervical cancer who are elderly, unmarried, and uninsured are more likely to be diagnosed at a late stage. These women should be targeted for cervical cancer education and screening programs.
INTRODUCTION
IN 1998 THERE were 13,700 estimated cases of invasive cervical cancer and 4900 deaths from cervical cancer in the United States.1 Stage at diagnosis is the most important prognostic determinant for invasive cervical cancer. Five-year survival is more than 90% for patients diagnosed as having localized disease, less than 50% for those with regional spread, and only 8% for those with distant disease.2 It is widely accepted that Papanicolaou (Pap) smear screening reduces the incidence and mortality from cervical cancer by detecting the cancer at an early or precancerous stage. Even with increasingly widespread use of the Pap test, almost half of all women with invasive cervical cancer are diagnosed as having disease at a late stage (regional or distant).2 More must be learned about factors affecting stage at diagnosis of cervical cancer to improve early detection of this potentially preventable and curable cancer.
The following factors have been shown in previous studies to be associated with later stage at diagnosis of cervical cancer: older age,3-5 black race or Hispanic ethnicity,6 and low education and income levels.6 These studies, however, did not control for confounding factors such as insurance type, comorbidity, or place of residence. Little is known about other possible predictors of late-stage diagnosis, such as a patient's smoking status, marital status, health insurance type, comorbidity, or residence in an urban or rural setting. Smoking increases the risk of cervical cancer and seems to have a promotional effect that might lead to a later stage at diagnosis.7-9 Smoking is also an independent risk factor for mortality within 5 years of presentation of cervical cancer.10 Unmarried women were more likely to be diagnosed as having advanced cervical cancer in one study,11 but not in others.5, 12 Among Medicare patients with cervical cancer, those enrolled in health maintenance organizations (HMOs) are less likely than fee-for-service enrollees to be diagnosed at a late stage.13 To our knowledge, no studies have examined the relationship of smoking, other health insurance types, comorbidity, or residence in an urban or rural setting to late stage at diagnosis of cervical cancer. By identifying predictors of late-stage diagnosis of cervical cancer, health education campaigns and interventions can be targeted toward certain populations. In addition, we can determine whether a specific form of health insurance defines a population at risk for late-stage cervical cancer.
This study used administrative data from Florida to determine clinical and demographic factors associated with stage at diagnosis of invasive cervical cancer. Our study controlled for age, race, education level, income level, smoking status, marital status, health insurance type, comorbid conditions, and residence in an urban or rural setting. We hypothesized that the following factors would be associated with greater odds of late-stage diagnosis: older age, nonwhite race, lower socioeconomic status, smoking, being unmarried, being uninsured, having other comorbid medical conditions, and living in a nonurban area.
MATERIALS AND METHODS
DATA SOURCES
We studied incident cases of invasive cervical cancer occurring in Florida during 1994 for which stage at diagnosis was available (N=852). Data from 1994 were used because this was the most recent year for which all relevant data were available. Incident cases were identified from the Florida Cancer Data System (FCDS), Florida's population-based statewide cancer registry. The FCDS was created in 1978 and has been collecting data on cancer incidence since 1981. The FCDS has well-established methods to ensure complete case finding, including cooperative arrangements with other state tumor registries, linkage with other databases, and ad hoc audits of reporting facilities. The development of cervical cancer proceeds along a continuum from precancerous stages (cervical intraepithelial neoplasia) to in situ cancers to frank invasive lesions. Invasive lesions are categorized by the FCDS as local (confined to the organ of origin), regional (cancers that have spread to regional lymph nodes or by direct extension beyond the organ of origin), and metastatic (lesions that have metastasized to distant sites). We had access to invasive lesions only because these are the only cancers reportable to the FCDS. In situ cervical cancers are not reportable to the FCDS and were therefore not included in our analysis.
The FCDS was the source for measures such as smoking status, marital status, age, race, and address of residence. To include information that is not routinely available from the FCDS (insurance payer, comorbidity, and socioeconomic status), cases were linked with state discharge abstracts and the 1990 US census. We obtained insurance payer and comorbidity information using state discharge abstracts. Comorbidity was determined using methods described by Charlson14 and Deyo15 and colleagues. The Charlson comorbidity index is not an exhaustive list of all possible comorbid conditions, but is rather a weighted index of 19 selected categories of disease that were found to be associated with mortality and other important health outcomes. Increasing scores on the Charlson comorbidity index reflect an increasing burden of comorbid conditions. We used International Classification of Diseases, Ninth Revision, Clinical Modification, mapping of comorbid conditions as described by Deyo et al.15 Cancer-related conditions were excluded. We used the original weights described by Charlson in calculating a morbidity index (theoretical range, 0-23). We defined 3 categories of comorbidity (0, 1, and 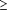 2) based on the patient's index score. Administrative data have generally proven accurate and valid in studies of cancer care.16-19 The 1990 US census was used to obtain aggregate measures of socioeconomic status. The FCDS provides the street address and ZIP code for each patient's residence. For 90% of patients, we were able to obtain the census tract of residence by geocoding their street address. For the remaining 10%, we used their ZIP code. Each individual was assigned the median income and education levels of the census tract (90% of patients) or ZIP code (10% of patients) of their residence. The use of census-derived measures of socioeconomic status has been validated in previous studies.20-23 This study was approved by the institutional review board of the University of South Florida, Tampa. 2) based on the patient's index score. Administrative data have generally proven accurate and valid in studies of cancer care.16-19 The 1990 US census was used to obtain aggregate measures of socioeconomic status. The FCDS provides the street address and ZIP code for each patient's residence. For 90% of patients, we were able to obtain the census tract of residence by geocoding their street address. For the remaining 10%, we used their ZIP code. Each individual was assigned the median income and education levels of the census tract (90% of patients) or ZIP code (10% of patients) of their residence. The use of census-derived measures of socioeconomic status has been validated in previous studies.20-23 This study was approved by the institutional review board of the University of South Florida, Tampa.
DATA ANALYSIS
The main outcome variable, stage at diagnosis, was defined using the SEER Site-Specific Summary Staging Guide.24 Stage at diagnosis is based on a combination of pathological, operative, and clinical assessments available within 2 months of diagnosis. For these analyses, stage at diagnosis was reclassified as either early (local) or late (regional and distant).
Variables assessed as possible predictors of late-stage diagnosis included age, race, marital status, current smoking status, median education level, median income level, comorbidity, urban residence, and insurance type. The relationship between predictor variables and likelihood of late-stage diagnosis was first assessed using the  2 test or Wilcoxon rank sum test as appropriate. The multivariate relationship between predictor variables and the odds of late-stage diagnosis were then examined using multiple logistic regression. Indicator variables were created for race (white and nonwhite), insurance type (Medicare, Medicaid, commercial HMO, commercial preferred provider organization, commercial indemnity, other insurance, and uninsured), smoking status (smoker and nonsmoker), marital status (married and nonmarried), comorbidity, and residence (urban and nonurban). We did not have sufficient sample size and statistical power to examine categories of nonwhite race. Commercial HMO insurance is defined by the FCDS as all HMO insurance plans other than HMOs provided by Medicare or Medicaid. 2 test or Wilcoxon rank sum test as appropriate. The multivariate relationship between predictor variables and the odds of late-stage diagnosis were then examined using multiple logistic regression. Indicator variables were created for race (white and nonwhite), insurance type (Medicare, Medicaid, commercial HMO, commercial preferred provider organization, commercial indemnity, other insurance, and uninsured), smoking status (smoker and nonsmoker), marital status (married and nonmarried), comorbidity, and residence (urban and nonurban). We did not have sufficient sample size and statistical power to examine categories of nonwhite race. Commercial HMO insurance is defined by the FCDS as all HMO insurance plans other than HMOs provided by Medicare or Medicaid.
The statistical significance of individual indicator variables was assessed using the  2 likelihood ratio test.25 To avoid forcing linear relationships with quantitative variables such as age, indicator variables were also created according to age categories.25 Relationships were then examined by graphing the corresponding odds ratios (ORs) for age categories.26-28 Linear relationships between age and the odds of late-stage diagnosis were subsequently tested in logistic models using the 2 likelihood ratio test.25 To avoid forcing linear relationships with quantitative variables such as age, indicator variables were also created according to age categories.25 Relationships were then examined by graphing the corresponding odds ratios (ORs) for age categories.26-28 Linear relationships between age and the odds of late-stage diagnosis were subsequently tested in logistic models using the  2 likelihood ratio test.25 Adjusted ORs and 95% confidence intervals (CIs) are reported. All reported P values are 2 tailed. Statistical significance was set at 2 likelihood ratio test.25 Adjusted ORs and 95% confidence intervals (CIs) are reported. All reported P values are 2 tailed. Statistical significance was set at  =.05. =.05.
RESULTS
The overall age-adjusted incidence rate of cervical cancer in Florida in 1994 was 10.5 cases per 100,000 population. The age-adjusted rate of late-stage cervical cancer was 4.84 cases per 100,000 population. Table 1 describes the characteristics of women diagnosed as having invasive cervical cancer in Florida in 1994. The mean (SD) age of patients having invasive cervical cancer was 52 (16) years (range, 21-96 years). There were notable numbers of patients who were nonwhite (including blacks and Hispanics), of low socioeconomic status, and uninsured. About 45% of patients with invasive cervical cancer had disease that had spread beyond the cervix.
|
|

|
|
Table 1. Characteristics of Women Diagnosed as Having Invasive Cervical Cancer in Florida in 1994
|
|
|
In bivariate analysis, patients who were diagnosed at a late stage were older than those diagnosed at an early stage (55.2 vs 48.4 years; t=6.4; P<.001). Table 2 presents the results of bivariate analysis. Greater likelihood of late-stage diagnosis was associated with being unmarried, having lower education and income levels, and having Medicare insurance or no insurance. Stage was not related to race, smoking, comorbidity, or place of residence. To examine the relationship between age and stage at diagnosis, ORs for age categories were graphed (Figure 1). The relationship appeared linear, with increasing age being associated with greater odds of late-stage diagnosis. Age was therefore modeled as a linear variable.
|
|

|
|
Table 2. Predictors of Late-Stage Cervical Cancer Diagnosis*
|
|
|
|
|

|
|
The relationship between patient age and the odds of late-stage cervical cancer diagnosis. Odds ratios are adjusted for age, race, marital status, education level, income level, smoking status, insurance type, comorbidity, and place of residence. The referent group is women younger than 40 years.
|
|
|
Multivariate predictors of late-stage cervical cancer at diagnosis are presented in Table 3. Age, marital status, and insurance type were the only variables in multivariate analysis to be significantly associated with late-stage diagnosis. Each additional year of age was associated with a 3% increased odds of late-stage diagnosis. Being unmarried was associated with a 63% increased odds of late-stage diagnosis. The unmarried group included never married (OR, 1.78; 95% CI, 1.15-2.78; P=.01), divorced (OR, 1.76; 95% CI, 1.11-2.79; P=.02), and widowed (OR, 1.27; 95% CI, 0.77-2.11; P=.35). Having commercial HMO insurance was associated with a 46% decreased odds of late-stage diagnosis compared with commercial fee-for-service insurance. Compared with patients having any form of health insurance, those who were uninsured had greater odds of late-stage diagnosis (OR, 1.60; 95% CI, 1.07-2.38; P=.02). Race, education level, income level, smoking status, comorbidity, and urban residence were not associated with stage at diagnosis in multivariate analysis. In stratified analysis, there were racial differences between urban and nonurban settings. There was a significant decreased odds of late-stage cervical cancer diagnosis among nonwhites in the nonurban setting (OR, 0.45; 95% CI, 0.23-0.90; P=.02). In the urban setting, there was a trend toward increased late-stage diagnosis among nonwhites that was not statistically significant (OR, 1.22; 95% CI, 0.73-2.02; P=.45). We examined 2-way interactions between insurance status and other demographic variables. Only one interaction was statistically significant, that between marital status and preferred provider organization insurance type (OR, 0.27; 95% CI, 0.10-0.75; P=.01). Being married reduced the odds of late-stage cervical cancer to a much greater extent among women having preferred provider organization insurance compared with women having fee-for-service insurance. The effects of marital status were the same for whites (OR, 0.60; 95% CI, 0.41-0.88; P=.009) and nonwhites (OR, 0.67; 95% CI, 0.35-1.30; P=.24).
|
|

|
|
Table 3. Multivariate Predictors of Late-Stage Cervical Cancer Diagnosis (N=730)*
|
|
|
COMMENT
Among women diagnosed as having invasive cervical cancer in Florida in 1994, more than 45% were diagnosed at a late stage. We found that increasing age, unmarried status, and being uninsured were independent risk factors for late-stage disease. Patients who had commercial HMO insurance were less likely to be diagnosed as having late-stage cervical cancer. There was a trend toward increased late-stage diagnosis among smokers who had cervical cancer that did not reach statistical significance. In contrast to our initial hypothesis, race, education, income level, comorbidity, and nonurban residence were not associated with stage at diagnosis of cervical cancer.
Results of our study support those of other studies that have found the elderly to be more likely to have late-stage invasive cervical cancer. This differs from breast cancer, in which younger age is associated with late-stage disease.29-30 Distinct from other studies, ours controlled for race, education level, income level, comorbidity, marital status, smoking status, and residence in an urban or nonurban area. The relationship between age and stage at diagnosis appeared linear, with increasing age being associated with greater odds of late-stage diagnosis. Women older than 65 years seem to be at highest risk of being diagnosed with late-stage cervical cancer. About 25% of invasive cervical cancers occur in women older than 65 years, yet this population accounts for more than 40% of the deaths.31 Several studies32-35 have shown that women older than 65 years are more likely to have never had a Pap test or to have not had one for several years. In addition, many elderly women who are diagnosed as having invasive cervical cancer have never had a Pap smear until diagnosis, yet 68% report having seen a physician in the preceding 3 years.36 Older women also tend to believe that they are less vulnerable to cervical cancer and that Pap tests are not worthwhile.37 In our study, even women in their 40s and 50s had increased odds of late-stage diagnosis of cervical cancer. It might be that as women age past their reproductive years and no longer need birth control or obstetric care, they may tend to see obstetrician-gynecologists and family physicians less often for pelvic examinations and Pap smears.
The US Preventive Services Task Force38 recommends Pap smears at least every 3 years for all women with a cervix who are or have been sexually active. Consideration can be made for discontinuation of testing after age 65 years if there was previous regular screening with consistently normal results. The American Academy of Family Physicians, the American Cancer Society, and the American College of Obstetricians and Gynecologists do not specify an upper age limit for Pap testing.39-41 The American College of Physicians recommends screening women 66 to 75 years of age every 3 years if they have not been screened within the 10 years before age 66 years.42 Some physicians may discontinue testing in patients older than 65 years without ensuring that there was previous regular screening. Costs of obtaining a Pap smear may be another barrier to testing for elderly women. However, it has been shown that cervical cancer screening in the elderly is cost-effective. Screening women older than 65 years every 3 years can reduce mortality from cervical cancer among the elderly by 74% at a cost of $2254 per year of life saved.43 Despite Medicare's reimbursing for Pap smears since 1990, the percentage of women undergoing Pap smear screening remained basically the same in 1987 and 1992.34 Physicians need to adhere to current guidelines and continue to screen women older than 65 years, especially if the women have not had regular screening with normal results.
This study also found that among women diagnosed as having cervical cancer, those who were unmarried were more likely to be diagnosed with late-stage disease compared with those who were married. Goodwin et al11 found similar results in a New Mexico population that included Hispanics and whites only. Their study could not be generalized to the black population. In studies by Nayeri et al12 and Mandelblatt et al5 that took place in metropolitan Brooklyn and New York City, respectively, and included data on blacks, unmarried persons were not at greater risk of diagnosis at a late stage for cervical cancer. Our study population included 69% whites and 31% nonwhites from the entire state of Florida. The effects of marital status did not seem to differ between whites and nonwhites. Married persons might enjoy more social and economic advantages and have better access to the health care system. The studies by Goodwin11 and Nayeri12 and their coworkers did not control for socioeconomic status or health insurance. In our study, after controlling for race, education, income level, type of health insurance, and comorbidity, marital status was still associated with stage at diagnosis. Unmarried women are less likely to ever have a Pap smear or have Pap smears in the past 3 years.32-33,35, 44 Unmarried women might not be currently sexually active and therefore less inclined to seek gynecologic care. The unmarried group also includes homosexual women who may mistakenly see themselves at lower risk for cervical cancer and not get routine Pap smears. Perhaps, married women receive more Pap smears because of greater contact with obstetrician-gynecologists during their childbearing years. After controlling for age, however, married women were still less likely to have late-stage cervical cancer. Married people have similarly been found to have earlier stages at diagnosis of breast,11 colon,45 and prostate12, 46 cancers. Married people might have better health habits and seek medical care sooner after the occurrence of symptoms of cervical cancer because of support and encouragement by their spouses.
Women who were uninsured were more likely to be diagnosed as having late-stage cervical cancer, whereas those who were enrolled in commercial HMOs were less likely to be diagnosed as having late-stage cervical cancer. Women insured by HMOs have the highest rate of Pap smear screening.47 On the other hand, women who are uninsured get fewer Pap smears than those who have insurance.33, 48 Uninsured persons are also more likely to be diagnosed with late-stage breast, colon, and prostate cancers.49
There was a trend for smokers with cervical cancer to be diagnosed as having more late-stage disease; however, it was not statistically significant given our sample size (only 250 patients were smokers). We also did not control for the amount or length of time a woman had smoked. Smoking has been associated with late-stage diagnosis of breast50-51 and prostate52-53 cancer. Further research is needed to determine whether smoking is an independent risk factor for stage at diagnosis of cervical cancer.
In contrast to results of other studies,5-6 race and socioeconomic status were not found to be associated with late-stage cervical cancer in our study. Education and income were related to late-stage diagnosis of cervical cancer in bivariate analysis but not in multivariate analysis, suggesting that they had no effect independent of the other variables in our model. Studies by Mandelblatt et al5 and Mitchell and McCormack6 included only metropolitan areas. Our study included urban and nonurban areas. In addition, our study controlled for comorbidity and insurance status. Outreach programs in Florida that have been targeted to minority women with low education and income and living in rural areas may have been successful in increasing Pap smear screening in this population.54-55 Nonwhite race and low socioeconomic status have been associated with late-stage diagnosis of breast49, 56 and prostate46, 49, 57 cancer but not colon cancer.49
We hypothesized that women with comorbid conditions would be more likely to be diagnosed as having late-stage cervical cancer because they are less likely to get Pap smears. In a recent study,58 each unit increase in comorbidity decreased Pap smear screening by 20%. In our study, comorbidity was not associated with late stage; however, 91% of the study population had no comorbid conditions. Further research is needed to determine whether comorbidity is an independent risk factor for stage at diagnosis of cervical cancer.
This study has several potential limitations. First, we relied solely on administrative data, the accuracy of which could not be independently verified. There are inherent limitations in using data from a state tumor registry. Most cases of cancer are reported to the tumor registry from hospitals. Patients who are never hospitalized may not be reported to the tumor registry. This should be less of a problem for invasive cervical cancer, which usually requires hospital services of some sort (surgery or radiation therapy). We were not able to assess earlier stages of cervical cancer, such as in situ, because those cases are not reportable to the tumor registry. The degree of detail in administrative data is also limited (eg, no differentiation between types of HMOs, cancer staging system not as complete as the TNM system, and incomplete list of comorbid conditions in discharge abstracts).
In addition, we relied on census-derived measures of socioeconomic status, which will not accurately reflect some patients' individual education or income.22 However, the use of census-derived measures of socioeconomic status has been validated in other studies. Associations between socioeconomic status and health outcomes have been similar when socioeconomic status is measured at the individual or community level.21-22 Some researchers20, 23 also argued that the socioeconomic level of a neighborhood is itself an important determinant of health. Our study was restricted to incident cases in Florida (having a higher proportion of elderly retired persons), so our findings might not be generalizable to other parts of the country. Finally, our study did not examine use of Pap smear screening in these women.
The results of this study suggest that elderly, unmarried, and uninsured women with cervical cancer are more likely to be diagnosed as having late-stage disease. Increasing Pap smear screening in these women is paramount to decreasing mortality from cervical cancer. Absence of a history of Pap smear is an independent risk factor for late stage at presentation of cervical cancer.10 Women with regional or distant invasive disease are least likely to have had Pap tests.10, 36 It has been shown that a physician's recommendation for screening can strongly influence patient behavior.59 Because elderly women are less likely than younger women to receive care from obstetrician-gynecologists,60 there is a need for family practice and general internal medicine physicians to recommend and perform Pap tests regularly in women older than 65 years. Unmarried and uninsured women should also be targeted for cervical cancer education and screening programs.
AUTHOR INFORMATION
Accepted for publication January 22, 2000.
This study was supported by Generalist Physician Faculty Scholars awards from the Robert Wood Johnson Foundation, Princeton, NJ (Dr Roetzheim).
Reprints: Jeanne Ferrante, MD, Department of Family Medicine, University of South Florida, 12901 Bruce B. Downs Blvd, MDC 13, Tampa, FL 33612.
From the Department of Family Medicine (Drs Ferrante, Gonzalez, Roetzheim, and Woodard and Ms Pal) and the H. Lee Moffitt Cancer Center and Research Institute (Drs Ferrante and Roetzheim), University of South Florida, Tampa.
REFERENCES
 |  |
1. Landis S, Murray T, Bolden S, Wingo P. Cancer statistics, 1998. CA Cancer J Clin. 1998;48:6-29.
ABSTRACT
2. Kosary C, Ries L, Miller B, Hankey B, Harras A, Edwards B. SEER Cancer Statistics Review, 1973-1992:Tables and Graphs. Bethesda, Md: National Cancer Institute; 1995.
3. Goodwin J, Samet J, Key C, Humble C, Kutvir D, Hunt C. Stage at diagnosis of cancer varies with the age of the patient. J Am Geriatr Soc. 1986;34:20-26.
ISI
| PUBMED
4. Free K, Roberts S, Bourne R, et al. Cancer of the cervix: old and young, now and then. Gynecol Oncol. 1991;43:129-136.
FULL TEXT
|
ISI
| PUBMED
5. Mandelblatt J, Andrews H, Kerner J, Zauber A, Burnett W. Determinants of late stage diagnosis of breast and cervical cancer: the impact of age, race, social class, and hospital type. Am J Public Health. 1991;81:646-649.
FREE FULL TEXT
6. Mitchell J, McCormack L. Time trends in late-stage diagnosis of cervical cancer: differences by race/ethnicity and income. Med Care. 1997;35:1220-1224.
FULL TEXT
|
ISI
| PUBMED
7. Licciardone JC, Wilkins III JR, Brownson RC, Chang JC. Cigarette smoking and alcohol consumption in the aetiology of uterine cervical cancer. Int J Epidemiol. 1989;18:533-537.
FREE FULL TEXT
8. Daling JR, Sherman KJ, Hislop TG, et al. Cigarette smoking and the risk of anogenital cancer. Am J Epidemiol. 1992;135:180-189.
FREE FULL TEXT
9. Prokopczyk B, Cox JE, Hoffmann D, Waggoner SE. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J Natl Cancer Inst. 1997;89:868-873.
FREE FULL TEXT
10. Bull AR, Hatton P, Bensley DC, Bull SJ, Fryers PT. Standardized mortality from cervical cancer: a measure of performance? J Public Health Med. 1994;16:16-22.
FREE FULL TEXT
11. Goodwin J, Hunt W, Key C, Samet J. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125-3130.
FREE FULL TEXT
12. Nayeri K, Pitaro G, Feldman J. Marital status and stage at diagnosis in cancer. N Y State J Med. 1992;92:8-11.
ISI
| PUBMED
13. Riley G, Potosky A, Lubitz J, Brown M. Stage of cancer at diagnosis for Medicare HMO and fee-for-service enrollees. Am J Public Health. 1994;84:1598-1604.
FREE FULL TEXT
14. Charlson M, Pompei P, Ales K, Mackenzie C. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383.
FULL TEXT
|
ISI
| PUBMED
15. Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619.
FULL TEXT
|
ISI
| PUBMED
16. Green J, Wintfeld W. How accurate are hospital discharge data for evaluating effectiveness of care? Med Care. 1993;31:719-731.
ISI
| PUBMED
17. Kahn LH, Blustein J, Arons RR, Yee R, Shea S. The validity of hospital administrative data in monitoring variations in breast cancer surgery. Am J Public Health. 1996;86:243-245.
FREE FULL TEXT
18. McClish DK, Penberthy L, Whittemore M, et al. Ability of Medicare claims data and cancer registries to identify cancer cases and treatment. Am J Epidemiol. 1997;145:227-233.
FREE FULL TEXT
19. Solin L, Legorreta A, Schultz D, Levin H, Zatz S, Goodman R. Analysis of a claims database for the identification of patients with carcinoma of breast. J Med Syst. 1994;18:23-32.
FULL TEXT
|
ISI
| PUBMED
20. Diez-Roux A. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88:216-222.
FREE FULL TEXT
21. Hofer T, Wolfe R, Tedeschi P, McMahon L, Griffith J. Use of community versus individual socioeconomic data predicting variation in hospital use. Health Serv Res. 1998;33:243-259.
ISI
| PUBMED
22. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703-710.
FREE FULL TEXT
23. Krieger N, Fee E. Social class: the missing link in U.S.health data. Int J Health Serv. 1994;24:25-44.
ISI
| PUBMED
24. Shambaugh E, Weiss M. Summary Staging Guide: Cancer Surveillance Epidemiology and End Results Reporting. Bethesda, Md: Public Health Service, National Institutes of Health, US Dept of Health and Human Services; 1977. Publication 86-2313.
25. Hosmer D, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons Inc; 1989.
26. Kleinbaum D, Kupper L, Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. Belmont, Calif: Lifetime Learning Publications; 1982.
27. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340-349.
FREE FULL TEXT
28. Rothman K. Modern Epidemiology. Boston, Mass: Little Brown & Co Inc; 1986.
29. Moritz D, Satariano W. Factors predicting stage of breast cancer at diagnosis in middle aged and elderly women: the role of living arrangements. J Clin Epidemiol. 1993;46:443-454.
FULL TEXT
|
ISI
| PUBMED
30. Yancik R, Havlik RJ, Wesley MN, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6:399-412.
FULL TEXT
|
ISI
| PUBMED
31. Brooks SE. Cervical cancer screening and the older woman: obstacles and opportunities. Cancer Pract. 1996;4:125-129.
PUBMED
32. Calle E, Flanders W, Thun M, Martin L. Demographic predictors of mammography and Pap smear screening in US women. Am J Public Health. 1993;83:53-60.
FREE FULL TEXT
33. The National Cancer Institute Screening Consortium for Underserved Women. Breast and cervical cancer screening among underserved women: baseline survey results from six states. Arch Fam Med. 1995;4:617-624.
FREE FULL TEXT
34. Anderson L, May D. Has the use of cervical, breast, and colorectal cancer screening increased in the United States? Am J Public Health. 1995;85:840-842.
FREE FULL TEXT
35. Lee J, Parsons GF, Gentleman JF. Falling short of Pap test guidelines. Health Rep. 1998;10:9-19.
36. Norman SA, Talbott EO, Kuller LH, et al. The relationship of Papanicolaou testing and contacts with the medical care system to stage at diagnosis of cervical cancer. Arch Intern Med. 1991;151:58-64.
FREE FULL TEXT
37. Lerman C, Caputo C, Brody D. Factors associated with inadequate cervical cancer screening among lower income primary care patients. J Am Board Fam Pract. 1990;3:151-156.
38. US Preventive Services Task Force. Guide to Clinical Preventive Services. 2nd ed. Bethesda, Md: US Dept of Health and Human Services; 1996.
39. American Academy of Family Physicians. Summary of Policy Recommendations for Periodic Health Examination. Kansas City, Mo: American Academy of Family Physicians; 1997.
40. American Cancer Society. 1999 Facts and Figures. Atlanta, Ga: American Cancer Society; 1999.
41. American College of Obstetricians and Gynecologists. Guidelines for Women's Health Care. Washington, DC: American College of Obstetricians and Gynecologists; 1996.
42. American College of Physicians. Screening for cervical cancer. In: Eddy D, ed. Common Screening Tests. Philadelphia, Pa: American College of Physicians; 1991:413-414.
43. Fahs MC, Mandelblatt J, Schechter C, Muller C. Cost effectiveness of cervical cancer screening for the elderly [see comments]. Ann Intern Med. 1992;117:520-527.
44. Skaer TL, Robison LM, Sclar DA, Harding GH. Cancer-screening determinants among Hispanic women using migrant health clinics. J Health Care Poor Underserved. 1996;7:338-354.
ISI
| PUBMED
45. Robinson E, Mohilever J, Zidan J, Sapir D. Colorectal cancer: incidence, delay in diagnosis and stage of disease. Eur J Cancer Clin Oncol. 1986;22:157-161.
FULL TEXT
|
ISI
| PUBMED
46. Ndubuisi S, Kofie V, Andoh J, Schwartz E. Black-white differences in the stage at presentation of prostate cancer in the District of Columbia. Urology. 1995;46:71-77.
47. Harlan L, Bernstein A, Kessler L. Cervical cancer screening: who is not screened and why? Am J Public Health. 1991;81:885-891.
FREE FULL TEXT
48. Hayward RA, Shapiro MF, Freeman HE, Corey CR. Who gets screened for cervical and breast cancer? results from a new national survey. Arch Intern Med. 1988;148:1177-1181.
FREE FULL TEXT
49. Roetzheim R, Pal N, Tennant C, et al. The effects of health insurance and race-ethnicity on the early detection of cancer. J Natl Cancer Inst. 1999;91:1409-1415.
FREE FULL TEXT
50. Daniell H. Increased lymph nodes metastases at mastectomy for breast cancer associated with host obesity, cigarette smoking, age, and large tumor size. Cancer. 1988;62:429-435.
FULL TEXT
|
ISI
| PUBMED
51. Scanlon EF, Suh O, Murthy SM, Mettlin C, Reid SE, Cummings KM. Influence of smoking on the development of lung metastases from breast cancer. Cancer. 1995;75:2693-2699.
FULL TEXT
|
ISI
| PUBMED
52. Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States). Cancer Causes Control. 1997;8:229-238.
FULL TEXT
|
ISI
| PUBMED
53. Coughlin SS, Neaton JD, Sengupta A. Cigarette smoking as a predictor of death from prostate cancer in 348,874 men screened for the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1996;143:1002-1006.
FREE FULL TEXT
54. McCoy CB, Nielsen BB, Chitwood DD, Zavertnik JJ, Khoury EL. Increasing the cancer screening of the medically underserved in south Florida. Cancer. 1991;67:1808-1813.
FULL TEXT
|
ISI
| PUBMED
55. Hopkins R. The Florida Cancer Plan. Tallahassee: Florida Cancer Control and Research Advisory Council; 1993.
56. Farley T, Flannery J. Late-stage diagnosis of breast cancer in women of lower socioeconomic status: public health implications. Am J Public Health. 1989;79:1508-1512.
FREE FULL TEXT
57. Delfino RJ, Ferrini RL, Taylor TH, Howe S, Anton-Culver H. Demographic differences in prostate cancer incidence and stage: an examination of population diversity in California. Am J Prev Med. 1998;14:96-102.
FULL TEXT
|
ISI
| PUBMED
58. Kiefe C, Funkhouser E, Fouad M, May D. Chronic disease as a barrier to breast and cervical cancer screening. J Gen Intern Med. 1998;13:357-365.
FULL TEXT
|
ISI
| PUBMED
59. Local data for local decision making—selected counties Connecticut, Massachusetts and New York, 1997. MMWR Morb Mortal Wkly Rep. 1998;47:809-813.
PUBMED
60. Teitelbaum MA, Weisman CS, Klassen AC, Celentano D. Pap testing intervals: specialty differences in physicians' recommendations in relation to women's Pap testing behavior. Med Care. 1988;26:607-618.
ISI
| PUBMED
RELATED ARTICLE
The Archives of Family Medicine Continuing Medical Education Program
Arch Fam Med. 2000;9(5):463-465.
FULL TEXT
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES
Medicaid Status and Stage at Diagnosis of Cervical Cancer
O'Malley et al.
AJPH 2006;96:2179-2185.
ABSTRACT
| FULL TEXT
Impact of Managed Care on Cancer Trial Enrollment
Gross and Krumholz
JCO 2005;23:3811-3818.
ABSTRACT
| FULL TEXT
Sicker and Poorer--The Consequences of Being Uninsured: A Review of the Research on the Relationship between Health Insurance, Medical Care Use, Health, Work, and Income
Hadley
Med Care Res Rev 2003;60:3S-75S.
ABSTRACT
Cervical Cancer: Disparities in Screening, Treatment, and Survival
Garner
Cancer Epidemiol. Biomarkers Prev. 2003;12:242s-247s.
FULL TEXT
|