
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
PROMISCUOUS LIGANDS AND ATTRACTIVE CAVITIES
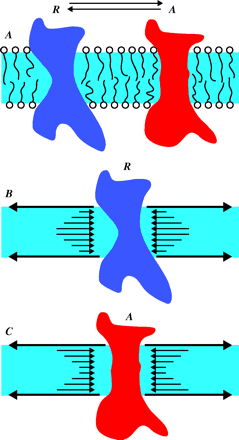
Lateral pressures within the membrane may control membrane protein conformational equilibria. Panel A shows the equilibrium between two conformational states (R for resting, and A for activated) of a hypothetical membrane protein. Panels B and C use arrows to represent the direction and magnitude of lateral pressure in a phospholipid membrane; the surface tension at the phospholipid/water interface is balanced by pressure of the opposite direction in the hydrophobic “core” of the membrane. The pressure in this core region is not constant across the bilayer; panel B shows that excess pressure in the middle will favor the R conformation of the protein, whereas the pressure profile represented in panel C will favor the A conformer. Changes in this lateral pressure profile are therefore expected to influence membrane protein conformational and insertion equilibria.


