
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
PROMISCUOUS LIGANDS AND ATTRACTIVE CAVITIES
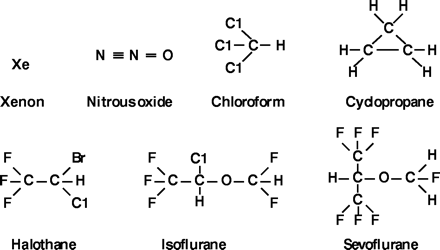
Figure 6.
Chemical structures of seven inhaled anesthetics. Two are no longer used clinically: chloroform, due to its toxicity; and cyclopropane, because it is explosive. Note the small differences in structure and size, small permanent dipole, and the lack of aromatics or good H-bond acceptors and donors. The more potent anesthetics (e.g., halothane) tend to have more polar functions.


