
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
PLC-ε: A Shared Effector Protein in Ras-, Rho-, and Gαβγ-Mediated Signaling
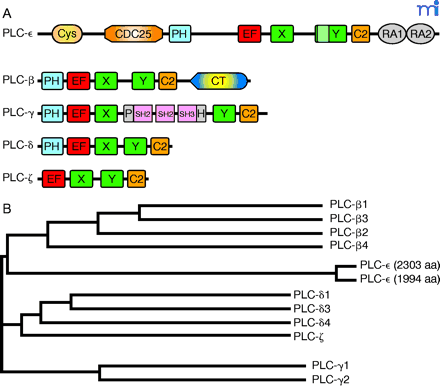
Domain organization and amino acid sequence comparisons among human PLC isozymes.
A. The five classes of phospholipase C isoforms are distinguished by differing structural domains, drawn here to relative scale in the full-length isozymes. The unique 60–70-residue “insert” is identified in the PLC-ε Y box.
B. A dendogram of the five groups of PLC isoforms is presented. The classification of PLC isoforms into five groups is reflected at the overall sequence level.
(Domain identification: Cys, cysteine-rich region; CDC25, guanine nucleotide exchange domain conserved among guanine nucleotide exchange factors (GEF); PH, pleckstrin homology domain; EF, EF-hand domain; X and Y, core catalytic domains (i.e., a TIM barrel); C2, Ca2+/lipid-binding domain; RA1 and RA2, Ras-associating domains. Genbank protein accession numbers for the known human PLC isozymes are as follows: PLC-ε, [1994 aa] AAG28341, [2303 aa] AAG17145; PLC-β1, Q9NQ66; PLC-β2, Q00722; PLC-β3, Q01970; PLC-β4, Q15147; PLC-γ1, P19174; PLC-γ2, P16885; PLC-δ1, P51178; PLC-δ3, NP_588614; PLC-δ4, NP_116115; PLC-ζ, NP_149114.)


