PLC-ε: A Shared Effector Protein in Ras-, Rho-, and Gαβγ-Mediated Signaling
- Address correspondence to TKH. E-mail tkh{at}med.unc.edu; fax 919-966-5640.
Abstract
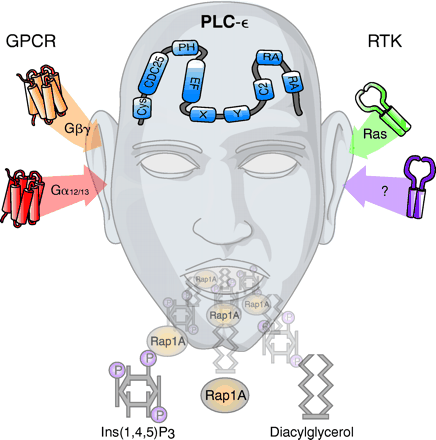
The conceptual segregation of G protein–stimulated cell signaling responses into those mediated by heterotrimeric G proteins versus those promoted by small GTPases of the Ras superfamily is no longer vogue. PLC-ε , an isozyme of the phospholipase C (PLC) family, has been identified recently and dramatically extends our understanding of the crosstalk that occurs between heterotrimeric and small monomeric GTPases. Like the widely studied PLC-β isozymes, PLC-ε is activated by Gβγ released upon activation of heterotrimeric G proteins. However, PLC-ε markedly differs from the PLC-β isozymes in its capacity for activation by Gα12/13 - but not Gαq -coupled receptors. PLC-ε contains two Ras-associating domains located near the C terminus, and H-Ras regulates PLC-ε as a downstream effector. Rho also activates PLC-ε , but in a mechanism independent of the C-terminal Ras-associating domains. Therefore, Ca2+ mobilization and activation of protein kinase C are signaling responses associated with activation of both H-Ras and Rho. A guanine nucleotide exchange domain conserved in the N terminus of PLC-ε potentially confers a capacity for activators of this isozyme to cast signals into additional signaling pathways mediated by GTPases of the Ras superfamily. Thus, PLC-ε is a multifunctional nexus protein that senses and mediates crosstalk between heterotrimeric and small GTPase signaling pathways.
- © American Society for Pharmacology and Experimental Theraputics 2003



