COPOLYMER 1: An Off-Beat Drug Development Story
Candace Pert, in a recent interview in Molecular Interventions, noted that she and her colleagues have been working on Peptide T for over seventeen years, and are just now planning Phase II clinical trials with this potential therapeutic agent for AIDS (1). This seemingly slow progress is reminiscent of the timeline of another peptide drug, which took seventeen years to reach Phase III testing and a total of thirty years to receive marketing approval in the United States. Copaxone®, also known as glatiramer, or Copolymer 1, received Food and Drug Administration (FDA) approval in December 1996 for treatment of the relapsing–remitting form of multiple sclerosis.
This article discusses the history of Copaxone® during its first two decades, prior to the involvement of Teva Pharmaceutical Industries, Ltd. of Israel and its American subsidiary, Lemmon Company of Sellersville, PA––an involvement which culminated in FDA approval of the drug. The story of Copaxone®, like that of Peptide T, is an atypical drug development story. It was given a slow but steady start by a group of persistent academic researchers in Israel who made a serendipitous observation and followed it up with exhaustive testing in animals. It then received strong forward impetus from an American research neurologist who produced, with no industry funding, a Phase III study which met all stringent requirements of the FDA. These are the most remarkable aspects of Copaxone® ’s development process.
One Drug, Three Names
The origins of each of the three names for this component are interesting. The drug consists of a mixture of polypeptides, but is not named as such. It was synthesized in a reaction that was essentially a copolymerization: four amino acids reacted to produce a mix of high molecular weight compounds. The original researchers named it Copolymer 1, the “1” indicating that it was the result of their first copolymerization reaction (2). Until 1996, the drug went through life as Copolymer 1, often shortened to Cop 1. Those familiar with the full name pronounced Cop as “cope”; others pronounced it as if talking about a police officer.
Teva, the firm which carried out the final steps of development of Cop 1 and filed a New Drug Application (NDA) for it, chose the trade name Copaxone®, which cleverly incorporated “Cop” and “one”, as well as including the word “axon” as a reference to the nervous system and the place at which the drug produces its beneficial action. However, it was also necessary to replace the trivial name Copolymer 1 with a name assigned by the United States Adopted Name (USAN) Council in Chicago. The Council produced the name glatiramer. The initials of the four constituent amino acids––glutamic acid, lysine, alanine, and tyrosine––were combined to form the first syllable; the “mer” ending is the standard USAN suffix for polymers. Thus, the USAN, or generic, name bears no relation to the name that appeared in the literature during the product’s entire developmental period.
Alphabet Soup: MS, MBP, and EAE
In tracing the origin of Copaxone®, one must look briefly at its target disease, multiple sclerosis (MS). This enigmatic, hard-to-diagnose disorder of the white matter of the central nervous system afflicts some 300,000 patients in the U.S.; approximately two-thirds of these patients are women. It typically strikes people in their twenties or thirties; it is not fatal, but causes ever-increasing disability, often culminating in complete helplessness. The two major types of the disease are classified as relapsing–remitting and chronic–progressive. The most common form, relapsing–remitting MS, begins with sensory disturbances (often, unilateral optic neuritis), limb weakness, ataxic gait, and neurogenic bladder and bowel symptoms. Remission from these attacks occurs, often speeded by administration of corticosteroids. Further relapses follow, which last longer and from which remission becomes increasingly more incomplete (3).
The etiology of MS has been baffling. None of the proposed theories have been proven; however, the myelin sheath in the spinal cord and the white matter of the brain are known to be attacked. The disease is thought to occur through an autoimmune process, but what triggers the autoimmune attack is not known (3).
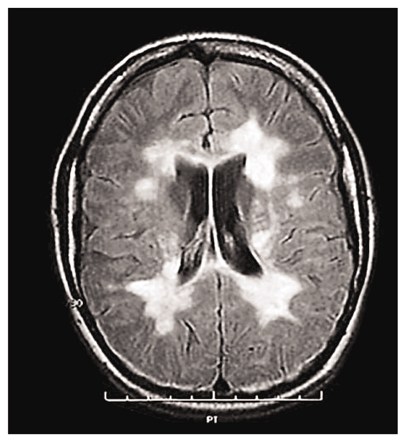
Myelin is a complex material whose main components are phospholipid (which renders it water- and ion-impermeable) and protein. About 40% of the total protein within myelin is myelin basic protein (MBP), which contains 170 residues in an open, double-stranded coil (4).
The animal model that most closely parallels MS is Experimental Allergic Encephalomyelitis (EAE). This condition can be induced in several mammalian species by a single injection of myelin-containing brain or spinal cord tissue in Freund’s adjuvant. It produces paralysis of the hind legs and, on autopsy, perivascular infiltration is observed in the brain. The active encephalitis-causing substance in the injected nervous tissue is MBP (5). As shown below, MBP was the key to the discovery of Copaxone®.
Discovery at the Weizmann Institute
In the early 1960s, Ruth Arnon, Michael Sela, and Dvora Teitelbaum at the biophysics department of the Weizmann Institute of Science, Rehovot, Israel, were working to determine the minimum chemical features necessary to make a molecule immunogenic, that is, to give it the ability to elicit antibodies in an animal. They attached short peptide chains of tyrosine, tryptophan, or phenylalanine to gelatin, and found that these derivatives had greater immunogenicity than unmodified gelatin. They then observed that the immunogenic properties resided entirely in the peptide moieties, and observed that a completely synthetic polypeptide with a molecular residual mass as low as 4000 daltons could be a potent antigen (6).
Turning their attention to the immunogenic EAE model, they reasoned that a polypeptide composed of amino acids found in MBP, and possessing a net positive electrical charge (as does MBP), might induce EAE similarly to the crude injections of myelin-containing tissue. To test this hypothesis they synthesized three random copolymers by reacting together various combinations of appropriately protected amino acids. The three copolymers were thus synthesized from the following amino acids:
Copolymer 1: alanine, glutamic acid, lysine, tyrosine (4 aa)
Copolymer 2: glutamic acid, lysine, tyrosine, glycine, histidine, proline, serine (7 aa)
Copolymer 3: alanine, glutamic acid, lysine, tyrosine, arginine, aspartic acid, glycine, histidine, leucine, phenylalanine, proline, serine (12 aa)
The reaction products were essentially mixtures of polypeptides of various chain lengths. The investigators were surprised to find that none of these products exhibited encephalitogenic activity; they did not induce EAE (2). On the contrary, further experiments showed them to be effective in suppressing EAE.
Using intradermal injections, in Freund’s adjuvant, all three had suppressant activity. However, the vehicle alone seemed active under these conditions, complicating the situation. Upon intravenous injection with saline, only Copolymer 1 protected the guinea pigs against EAE and did not produce toxic effects. Interestingly, it was the simplest of the copolymers––the one consisting of only four amino acids––that showed activity. Similar results in guinea pigs were obtained when EAE was induced using MBP from bovines or humans. Additionally, Copolymer 1 also ameliorated EAE induced in other species (e.g., rabbits, mice, rhesus monkeys, and baboons) (5, 7).
Having convinced themselves that, instead of a pharmacologic tool, they had a drug candidate, the Weizmann group proceeded to perform further preclinical testing. Acute or subchronic administration in mice, rats, rabbits, and beagle dogs showed Copolymer 1 to be nontoxic and not taken up significantly by any of the animal organs. In view of the putative resemblance between EAE in animals and MS in humans and the hypothesis that MBP may be involved in the pathogenesis of MS, the decision to conduct preliminary clinical trials was made. First, Copolymer 1 was administered to four patients with advanced MS, suffering from acute disseminated encephalomyelitis. This was followed by an open study in four patients with relapsing–remitting MS and twelve with the chronic–progressive form of the disease. The results of both preliminary trials were sufficiently promising to warrant a double-blind, controlled clinical trial (5).
Bornstein Brings Copolymer 1 to the US
The American professor of neurology and neuroscience Murray B. Bornstein dominates the next part of the story. Born in 1908 in Paterson, New Jersey, Bornstein graduated from Dartmouth College in 1939. In 1952, he received the MD degree from the University of Geneva. From 1956 to 1958, he was a visiting fellow at the College of Physicians and Surgeons of Columbia University, and served as laboratory director at Mount Sinai Hospital in Manhattan from 1958 to 1966. He then joined the faculty of the Albert Einstein College of Medicine of Yeshiva University in Bronx, New York, from which he formally retired in 1988 as a professor in the Departments of Neurology and Neuroscience. After his retirement, Bornstein remained active at Albert Einstein until 1994. In an ironic coincidence, he died soon after giving court testimony as an expert witness in a case involving MS (8). At the time of his death, Copaxone® was only fifteen months away from FDA approval.
Bornstein’s indispensable contribution to Copaxone® ’s drug development process lay in organizing and acting as principal investigator of a pilot study on fifty patients with relapsing–remitting MS. Half the patients were treated with the active copolymer dissolved in saline, and half with saline alone (placebo). Patients were taught to self-inject the solution, and had to do so daily for two years. Patients were evaluated every three months, and also whenever a suspected exacerbation (relapse) occurred. The principal endpoint was the proportion of exacerbation-free patients. Secondary endpoints were 1) the frequency of exacerbations, 2) the change in patients’ disability scores from their baselines, and 3) the length of time before disease progression. Endpoints and statistical methods were spelled out in advance. The study was conducted under an Investigational New Drug (IND) application, meaning that the FDA reviewed the protocol and supporting data in advance, and permitted the trial to proceed.
The study was quite difficult to manage because of the complex nature of both drug and disease. The stability of Copolymer 1 at ambient temperature, or even under refrigeration, was unknown, so the active and placebo solutions were stored frozen. The patients received their supplies in the frozen state, stored them in their freezers, and had to let them thaw to room temperature before injecting. The examining neurologists were kept blinded to the drug assignment. Side effects and change in neurological status were reported separately to a non-blinded clinical assistant.
When the trial was completed, 56% of the patients on active product suffered no relapses, compared with 26% of the patients on placebo. The frequency of relapses over two years in the placebo group vs the Copolymer 1 group averaged 2.7 and 0.6 per patient, respectively. Patients taking Copolymer 1 had reduced disability on average, while placebo patients worsened. Fifty percent of those in the placebo group showed disease progression by the end of eighteen months, while only 20% of the Copolymer 1 group had progression at the end of twenty-four months. These results were statistically significant. Adverse effects were largely limited to irritation at injection sites and the rare occurrence of a transient vasomotor response (9). Clearly, the results warranted further evaluation of Copolymer 1 in a full-scale multicenter clinical trial.
There were two remarkable things about this study. First, it received no industrial support; being funded, instead, by grants from the National Institutes of Health and the National Institute of Neurological and Communicative Disorders and Stroke [now the National Institute of Neurological Disorders and Stroke (NINDS)]. The drug and placebo supplies were furnished by the Weizmann Institute and a small company associated with Weizmann which played no further role in the drug’s development. Second, the study was of sufficient quality to be accepted by the FDA as one of the two pivotal Phase III clinical trials. For a study to be so accepted––being published in a peer-reviewed journal (9) as this one was––is not enough. The underlying documentation, from the information provided to patients to gain their informed consent to the patient records accumulated throughout the study, must stand up to FDA scrutiny. The documentation may be inspected by the FDA at the trial site either during or after the study, and it is certainly reviewed when submitted as part of the NDA. Typically, an industrial sponsor sends clinical research monitors to guide the medical personnel and assure compliance with good clinical practices. Greatly to the credit of Murray Bornstein and his team of associates, this study passed muster despite the absence of any pharmaceutical firm’s involvement.
In Conclusion
The development saga of Copolymer 1 did not end there. Following the publication of Bornstein’s study in 1987, a pharmaceutical firm that made a formidable commitment of funds and staffing took on further development of Copolymer 1. It took nearly a decade to perform a second (multicenter) clinical trial, complete the necessary work on the chemical manufacturing process and control procedures, assemble a New Drug Application (NDA), and shepherd the product through the FDA review process. Today, Copaxone® is an important tool in the struggle against MS; by virtue of its unique chemistry, it is the only such MS-targeted product that is not an interferon (10). The story of Copaxone® shows how far academic researchers can move a potential medicinal agent. It is a tribute to the biological, chemical, and medical investigators who shaped the first twenty years of its history.
- © American Society for Pharmacology and Experimental Theraputics 2004
References

Stanley Scheindlin, DSc, RPh, holds graduate degrees in pharmaceutical chemistry and worked in drug product development and regulatory affairs. Now retired, he is a part-time consultant and writes freelance articles for pharmacy-related specialty publications.