
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Fibrin Mechanisms and Functions in Nervous System Pathology
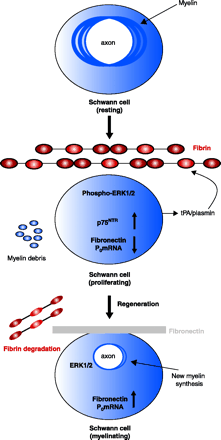
Proposed mechanism for the effects of fibrin in peripheral nerve remyelination. Myelinating Schwann cells in the adult normal sciatic nerve have established axonal contacts and produce myelin that enwraps the axons. After injury, axons degenerate, myelin is degraded, and fibrin is deposited. Fibrin deposition regulates Schwann cell numbers by increasing the phosphorylation of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and by increasing the protein expression of p75 neurotrophin receptor (p75NTR). The combined action of the proliferating ERK1/2 pathway and the p75NTR pathway sustains the Schwann cells in a nonmyelinating state, as evidenced by the shut down of myelin genes transcription [e.g., the myelin protein zero (P0) gene], and decreased amounts of fibronectin transcripts. Downregulation of the P0 or the fibronectin gene could either be due to the increased ERK1/2 phosphorylation as observed in transformed cells (144), and/or by another mechanism. When fibrin is degraded during nerve regeneration, ERK1/2 becomes unphosphorylated, p75NTR levels decrease, fibronectin is produced and deposited in the nerve, and Schwann cells transcribe myelin genes and remyelination is initiated. Up-regulation of P0 associated with downregulation of p75NTR is a characteristic change associated with myelination (145). Reprinted from (66); Neuron, 14, Akassoglou et al. “Fibrin inhibits peripheral nerve remyelination by regulating Schwann cell differentiation,” 861–875, (2002), with permission from Elsevier.


