Transdermal Drug Delivery: PAST, PRESENT, FUTURE
Delivering medicine to the general circulation through the skin is seen as a desirable alternative to taking it by mouth. Patients often forget to take their medicine, and even the most faithfully compliant get tired of swallowing pills, especially if they must take several each day. Additionally, bypassing the gastrointestinal (GI) tract would obviate the GI irritation that frequently occurs and avoid partial first-pass inactivation by the liver. Further, steady absorption of drug over hours or days is usually preferable to the blood level spikes and troughs produced by oral dosage forms.
These advantages are offered by the currently marketed transdermal products. One of the most successful, the nicotine patch, releases nicotine over sixteen hours, continuously suppressing the smoker’s craving for a cigarette. The scopolamine patch is worn behind the ear and releases the alkaloid for three days, preventing motion sickness without the need to swallow tablets periodically. The fentanyl patch acts for seventy-two hours, providing long-lasting pain relief. And an estrogen–progestin contraceptive patch needs to be applied only once a week, a boon for women who find it onerous to take one pill every day.
The transdermal route is indeed desirable, but there is one small obstacle: whereas the function of the GI tract is to render ingested material suitable for absorption, the skin’s function is to keep things out of the body. The major barrier within the skin is the stratum corneum, the top layer of the epidermis. The stratum corneum consists of keratinized, flattened remnants of once actively dividing epidermal cells (1). Hygroscopic, but impermeable to water, it behaves as a tough, flexible membrane. The intercellular space is rich in lipids. The stratum corneum is about ten microns thick, but on the palms and soles it ranges up to 600 microns in thickness (1).
Although the stratum corneum is an efficient barrier, some chemical substances are able to penetrate it and to reach the underlying tissues and blood vessels. These “successful” substances are characterized by low molecular weight (≤500 Da), lipophilicity, and effectiveness at low dosage. The largest daily dose of drug in patch form is that of nicotine: twenty-one milligrams (Table 1⇓).
Characteristics of Several Drugs Delivered Transdermally
Transdermal absorption occurs through a slow process of diffusion driven by the gradient between the high concentration in the delivery system and the zero concentration prevailing in the skin. Thus, the delivery system must be kept in continuous contact with the skin for a considerable time (hours to days).
Current Transdermal Products
The major products currently marketed for transdermal absorption are the transdermal therapeutic systems (TTS), popularly known as patches (Table 1⇑) (2). The functional parts of a patch, proceeding from the visible surface inward to the surface apposed to the skin, are:
-
An impermeable backing
-
A reservoir holding the active ingredient, together with release-controlling materials
-
An adhesive to hold the patch in place on the skin
-
A protective cover that is peeled away before applying the patch
Most patches belong to one of two general types – the reservoir system, and the matrix (or drug-in-adhesive) system.
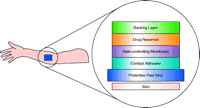
A schematic drawing of the scopolamine patch (Table 1⇑), as an example of the reservoir system, is provided in Figure 1⇓ (3). The impermeable backing is the visible surface of the patch after application. The protective peel strip is removed before applying the patch. The drug is dispersed in liquid excipients––inactive compounds in the liquid vehicle––in the reservoir. The rate-controlling membrane is made of micro-porous polypropylene and controls the rate at which scopolamine is transferred from the reservoir to the skin surface. The adhesive layer contains some scopolamine in addition to the adhesives.
The layers that comprise the reservoir type of patch. The protective peel strip is removed prior to applying the patch to skin.
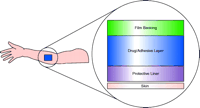
Climara ® (estradiol) is a typical patch of the matrix type (Figure 2⇓) (4). Here the active ingredient is dispersed entirely in the adhesive. The protective liner is removed before applying, and the film backing remains when the patch is applied. The excipients in both types of patches are unlike those found in traditional topical products such as ointments, creams, and lotions. The backing layer on both patches is made of polyester film, ethylene vinyl alcohol copolymer (EVA), or polyurethane film. The removable strip is often composed of polyester fabric. The adhesive is generally an acrylic polymer, and polyisobutylene is a frequent component of both the adhesive and the drug reservoir. The technology of the backing layer and the removable layer is related to that of the Band-Aid ®–type protective strip.
The layers that comprise the matrix (drug-in-adhesive) type of patch. The protective liner is removed prior to applying the patch to skin.
Developing a patch formulation is a complex process. The rate and amount of transdermal absorption depend on many factors, including the nature of the drug, the drug’s concentration in the reservoir or matrix, and the area of skin covered by the patch. When several dose-strengths of a drug patch are marketed (e.g., estradiol patches), the formulations used are identical but the patches have different surface areas for different strengths of delivered drug. A large excess of drug is placed in the patches to keep the concentration gradient favorable for absorption. For example, the labeling of the lidocaine patch (Lidoderm®) states that only 3% of the applied dose is absorbed (5). Because the active ingredients act at low dosage and are inexpensive, the cost of wasted excess drug is not economically significant.
Very few companies, even among “big pharma,” possess the know-how and equipment to deal with the unusual ingredients involved, to work out the parameters needed to obtain the desired absorption rates, and to assemble the patches. Therefore, development and manufacture of these products are generally outsourced.
Avoidance of the need to contract out is probably the reason why a few transdermals are still marketed as ointments or gels. One example is Androgel® (testosterone 1%) in which the testosterone is dissolved in alcohol (69%) and water. The liquid is gelled with Carbomer, a vinyl polymer containing active carboxyl groups. When the liquid-gel is subsequently neutralized with sodium hydroxide, the hydroalcoholic solution becomes a semisolid. Five-gram quantities of Androgel® are individually packaged. The patient squeezes the contents into his hand and applies once daily to specific areas of the skin (6).
The disadvantages of transdermal gels or ointments are obvious. A relatively large amount must be applied, the area of contact is variable, and the hands must be washed thoroughly to remove residual potent substances such as testosterone or nitroglycerin.
Historical Perspecitve
Transdermal delivery of medications was foreshadowed in earlier eras by the use of certain plasters and ointments. The mustard plaster, applied as a home remedy for severe chest congestion, may be considered an example. (This author remembers his mother making mustard plasters for members of the family.) Powdered mustard seed (Brassica nigra) was mixed with warm water, and the resulting paste was spread on a strip of flannel, which was applied to the patient’s chest with a cloth binding wrapped around the body to hold the plaster in place. The moisture and body warmth activated an enzyme (myrosin) in the mustard that hydrolyzed a glycoside (sinigrin), causing the release of the pungent active ingredient allyl isothio-cyanate (CH2=CHCH2NCS) (7). This substance possesses the qualifications listed above for transdermal absorption.
It is a low molecular weight liquid (90 Da), lipophilic, and effective at low dosage. The flannel served as an impermeable backing and the mustard paste was the reservoir. Continuous enzymatic action released the active substance over a period of hours, until the plaster was removed. Commercially manufactured mustard plasters were sold at pharmacies.
The history of plasters has been traced back to antiquity. In addition to mustard plasters, several other plasters were recognized in early 20th century editions of the United States Pharmacopeia (USP) and National Formulary (NF). At one time, Belladonna Plaster, containing 0.25–0.30% of belladonna root alkaloids, was believed to act transdermally as an analgesic (8).
Perhaps the most remarkable forerunner of modern transdermal medication was Stronger Mercurial Ointment, used as a treatment for syphilis when Salvarsan and other arsenicals were in use, before the discovery of penicillin. Even the composition of this ointment was remarkable; it contained 50% of elemental mercury! To make the ointment, liquid mercury was first mixed with mercuric oleate, a mercury soap. Dispersion of the mercury into microscopic globules required a large input of energy, either by hand trituration with mortar and pestle, or by a power mixer. The dispersed mercury was made into an ointment with benzoinated lard, using suet as a stiffening agent. Stronger Mercurial Ointment was applied and rubbed into areas where the skin is thinnest: the groin and the bends of the elbows and knees. The amount applied was two to four grams of ointment, containing one to two grams of mercury.
At the time this product was in use, questions were raised about its mode of action. It was suggested that instead of mercury being absorbed through the skin, the patient was actually inhaling mercury vapor. Schamberg conducted a study in rabbits and concluded that the bulk of mercury absorption was transdermal (9). A more definitive study, in humans, was reported by Cole, Gericke, and Sollmann (10). After the ointment was rubbed in, excess ointment was washed off the skin with solvent, eliminating any source of mercury vapor. In this study, 75% of the subjects experienced salivation, one of the known systemic effects of mercury. This study was essentially a crude bio-availability study based on a clinical endpoint.
The Future of Transdermal Therapy
Ten years ago, the nicotine patch had revolutionized smoking cessation; patients were being treated with nitroglycerin for angina, clonidine for hypertension, scopolamine for motion sickness, and estradiol for estrogen deficiency, all through patches. At that time, biotech medicinals were still being developed. During the past decade biotech products have come into their own, but transdermals have essentially remained static. The number of drugs formulated in patches has hardly increased, and there has been little change in the composition of the patch systems. Modifications have been mostly limited to refinements of the materials used (11). One reason for this undoubtedly is the fact that only certain specialized firms can manufacture transdermal patches. Companies prefer to have full control of their projects, and to enjoy the higher profits on products developed and manufactured in house. Another reason is that only a limited number of drugs fit the molecular weight, lipophilicity, and potency requirements for transdermal absorption.
Molecular Absorption Enhancement
Considerable research has been done on absorption enhancers, compounds that promote the passage of drugs through the stratum corneum. Terpene derivatives as well as certain phenols seem to improve transdermal absorption. For example, linalool, alpha terpineol, and carvacrol were studied in conjunction with haloperidol (a commonly prescribed neuroleptic drug). All three enhanced haloperidol absorption, but only linalool increased it to a therapeutic level (12). Limonene, menthone, and eugenol were found to enhance transdermal absorption of tamoxifen (13). Phloretin, a polyphenol, enhanced the absorption of lignocaine (14). In general, absorption enhancement research has been done with excised animal skin (pig or rabbit) or human skin obtained from cadavers or plastic surgery procedures.
In contrast, an interesting clinical trial was reported from Australia (15) where estradiol was formulated as a metered-dose aerosol, using padimate O [a para-aminobenzoic acid (PABA) derivative used as a sunscreen agent] as the penetration enhancer. The volunteer subjects were four healthy, postmenopausal women. The aerosol was applied to the ventral forearm in three sprays, each delivering one milligram of estradiol. Each spray covered 10 cm2 of skin. After administering the spray, the skin was not touched for two minutes, but then normal activity, including washing and dressing, was resumed. The drug was applied in this way for nine successive days. Plasma estradiol/estrone ratios obtained for the topical aerosol were consistent with those produced by a topical gel and a transdermal patch, showing that a clinically relevant dose of estradiol was delivered. Using the Draize skin irritation test, no irritation was observed.
This dosage form appears to be a practical alternative to the patch, unless inadvertent inhalation of the spray turns out to be a problem.
Absorption Enhancement by Energy Input
The above are potential adjuvants to the existing “passive” transdermal systems. Also under study is the possibility of active transfer of drugs through the skin by the action of electrical or other forms of energy. The most research has been devoted to iontophoresis; sonophoresis and electroporation have been less well studied.
Iontophoresis is a method of transferring substances across the skin by applying an electrical potential difference. It promotes the transfer of charged ionic drugs and possibly high molecular weight substances such as peptides. Electric current is applied through two electrodes, placed on the patient’s skin. The first, or donor, electrode (cathode) delivers the negatively charged therapeutic agent (e.g., an organic acid), whereas the second, or receptor, electrode (anode) serves to close the circuit. This setup is named cathodal iontophoresis. For positively charged drugs (e.g., amines or peptides), the cell arrangement is reversed (anodal iontophoresis). The silver (anode) and silver chloride (cathode) electrode system—utilized in both types of iontophoresis––is favored largely because it does not affect the drug solution to the extent that other electrode systems can (16).
Current commercial applications of iontophoresis include intradermal administration of lidocaine as a local anesthetic and dexamethasone for local inflammation. The devices used are typically bench-top systems with patches connected to a power supply through cables; however, innovations in electronic circuit and battery technology may make small, integrated patch-like systems practicable (16).
A small number of human trials have been conducted as proof of concept. Thus, fentanyl was tested in twelve healthy volunteers who were protected from its opioid effects by naltrexone administration. Analysis of plasma levels of fentanyl revealed transdermal absorption. Luteinizing hormone-releasing hormone (LHRH), a decapeptide having a molecular weight of about 1200 Da, was tested in eight healthy male volunteers, demonstrating that pulsatile delivery of this hormone is feasible (16).
Electroporation is a technique that delivers high voltage pulses of micro-to-millisecond duration to the skin, causing transient changes in cell membranes or lipid bilayers (17). It is hypothesized that pretreatment of the skin in this way would enable the passage of large, polar molecules such as heparin and peptides.
Low frequency ultrasound treatment, or sonopheresis, was reported to enhance absorption of mannitol, which was used as a model for highly hydrophilic drugs (18).
A Potential Breakthrough?
The literature cited in this pick-and-choose historical article on both the molecular and energetic enhancement of trans-dermal penetration is of academic interest, but has not resulted in new products. However, a breakthrough might have occurred recently. At a recent meeting of the American Society of Plastic Surgeons, a firm announced the market introduction of its new ActiPatch TM Therapy––a dermal patch with an embedded microchip that delivers weeks of continuous therapy. The patch is described as the miniaturized equivalent of the pulsed electromagnetic energy machines long used by physicians to reduce swelling, relieve pain and enhance healing of surgical incisions, accidental wounds and bedsores (19). Although this patch does not deliver drug therapy, the fact that a microchip can act as a continuous source of energy may be applicable in the future to iontophoresis. Thus, we may see major advances in the currently stagnant field of transdermal drug delivery.
- © American Society for Pharmacology and Experimental Theraputics 2004
References

Stanley Scheindlin, DSc, RPh, holds graduate degrees in pharmaceutical chemistry and worked in drug product development and regulatory affairs. Now retired, he is a part- time consultant and writes freelance articles for pharmacy-related specialty publications.