Increased Expression and Function of Vascular α1D-Adrenoceptors May Mediate The Prohypertensive Effects Of Angiotensin II
Angiotensin II (Ang II) is synthesized by the renin-angiotensin system (RAS) and participates in regulating systemic arterial
pressure. Ang II acts as a potent vasoconstrictor to activate Ang II type 1 (AT1) receptors on vascular smooth muscle and affects cardiac and vascular remodeling, cardiac contractility, and pulse rate through
increased sympathetic nervous system tone (by promoting presynaptic facilitatory modulation of noradrenaline release) (1). Physiological parameters regulated by RAS, such as plasma renin activity, plasma angiotensinogen concentration (2, 3), and kidney renin release (3, 4) are known to be elevated in young spontaneously hypertensive rats (SHR), suggesting that they might contribute to the pathogenesis
of genetic hypertension.
Is there crosstalk between RAS and adrenergic peripheral pathways in the genesis of hypertension? Schiffrin and coworkers
(5) observed an increased density of α1-adrenoceptors and Ang II receptors in the vasculature of four-week-old SHR before the development of hypertension. The stimulatory
effect of Ang II on smooth muscle cell DNA synthesis in vivo was markedly decreased by cotreatment with the α1-adrenoceptor antagonist prazosin (6). Another study demonstrated that high doses of captopril, an Angiotensin Converting Enzyme inhibitor, inhibited the abnormal
hypersensitivity of resistance vessels to phenylephrine, an α1-adrenoceptor agonist (3, 7). Moreover, RAS blockade by pharmacological means with ACE inhibitors or AT1 receptor antagonists in young SHR may attenuate or even prevent the development of hypertension (8–12).
On the other hand, Hoffman and coworkers demonstrated that Ang II induces α1-adrenoceptor expression, mainly α1D- subtype, in isolated rat aorta smooth muscle cells (13), and Faber and colleagues elegantly showed that α1D-adrenoceptor activation increased protein synthesis in arterial smooth muscle (14). These data suggest that Ang II may facilitate aorta smooth muscle hypersensitivity and hypertrophy through α1D-adrenoceptors expression.
Several arteries in SHR functionally express α1D-adrenoceptors, and once stimulated, these receptors mediate contraction (15–17). In addition, increasing evidence has revealed that vascular α1D-adrenoceptors are functionally important for the genesis and/or maintenance of hypertension: the receptors appear to be present
prior to the establishment of hypertension and their effect increases with aging in SHR. Also, an augmented population of
constitutively active α1D-adrenoceptors might be responsible for the pathologic consequences of sympathoadrenal-mediated increased smooth muscle tone
in SHR (8, 15–20).
Tsujimoto and coworkers showed that genetic disruption of the α1D-adrenoceptor gene generates hypotensive mice, suggesting that these receptors are important for blood pressure control (21). Similarly, D’Ocon’s group reported that a constitutively active α1D-adrenoceptor population (putatively involved in the pathology of the SHR) functionally disappeared in arteries where that
subtype predominates for contraction, after a long term and high dose of captopril therapy (8), further implicating α1D-adrenoceptor in pathological hypertension. We have found that prehypertensive SHR have augmented basal amounts of α1D-adrenoceptor mRNA and protein as compared to those amounts observed in normotensive Wistar Kyoto rats. These data suggest
that the Ang II and α1D-adrenoceptor systems might impinge upon each other in the onset of hypertension. Thus, we hypothesize that Ang II facilitates
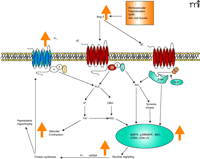
hypertension through stimulation of vascular α1D-adrenoceptor expression and function (Figure 1⇓) and that this specific adrenoreceptor may mediate blood vessel hypertrophy and hypersensitivity.
The use of aryl hydrocarbon receptor (Ahr) knockout mice—which exhibit hypertension and cardiac hypertrophy, and have higher
than normal circulating concentrations of Ang II and endothelin 1(22)—should support our hypothesis. Ahr-deficient mice express α1D-adrenoceptors for aortic smooth muscle contraction. Aorta from Ahr-deficient mice will likely respond with a greater maximal
contraction to α1-agonists, owing to elevated Ang II—mediated increased expression of α1D-adrenoceptors as compared to the contractile effects and amounts of α1D-adrenoceptors found in wild-type mice aortas.
Figure 1.
Suggested signal transduction mechanism involving Ang II-AT1 receptor and its influence on α1D-adrenoceptor expression and function. In the orange box are conditions where Ang II levels are elevated, which favor increased α1D-adrenoceptor expression and function. PLC, phospholipase C; PKC, protein kinase C; β-arr, β-arrestin; GRK, G protein receptor
coupled kinase; Src, a tyrosine kinase.
Acknowledgments
Authors thank PAPIIT IN230205 (RV-M), IN210702 (MI) and Fundación Miguel Alemán (RV-M) for support grants.
- © American Society for Pharmacology and Experimental Theraputics 2005
References
- ↵
Weir, M.R. and Dzau, V.J. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am. J. Hypertens. 12, 205S–213S (1999).
- ↵
Ruiz, P., Basso, N., Cannata, M.A., and Taquini, A.C. The renin-angiotensin system in different stages of spontaneously hypertension
in the rat (SHR). Clin. Exp. Hypertension: Theory and Practice A 12, 63–81 (1990).
- ↵
Zicha, J. and Kune, J. Ontogenetic aspects of hypertension development: Analysis in the rat. Physiol. Rev. 79, 1227–1282 (1999).
- ↵
Henrich, W.L. and Levi, M. Ontogeny of renal renin release in spontaneously hypertensive rat and Wistar-Kyoto rat. Am. J. Physiol. 260, F530–F535 (1991).
- ↵
Schiffrin, E.L., Thome, F.S., and Genest, J. Vascular angiotensin II receptors in SHR. Hypertension 6, 682–688 (1984).
- ↵
Van Kleef E.M., Smits, J.F.M., De Mey, J.G.R., Cleutjens, J.P.M., Lombardi, D.M., Schwartz, S.M., and Daemen, M.J.A.P. α1-adrenoceptor
blockade reduces the angiotensin II-induced vascular smooth muscle cell DNA synthesis in the rat thoracic aorta and carotid
artery. Circ. Res. 70, 1122–1127 (1992).
- ↵
Chen, D.G., Jin, X.Q., Wang, H.J., and Chen, S.C. Mechanisms responsible for sustained hypotension after captopril treatment.
J. Hypertension 13, 1113–1121 (1995).
- ↵
Gisbert, R., Ziani, K., Miquel, R., Noguera, M.A., Ivorra, M.D., Anselmi, E., and D’Ocon, P. Pathological role of a constitutively
active population of α1D-adrenoceptors in arteries of spontaneously hypertensive rats. Br. J. Pharmacol. 135, 206–216 (2002). This article shows that therapy with captopril (50 mg/kg/day/10 weeks) inhibits the increase in resting tone (IRT) response,
a measure of α1D-adrenoceptor constitutive activity, in both WKY and SHR rats and suggests that IRT is not observed because SHR rats did
not become hypertensive.
-
Harrap, S.B., Van Der Merge, W.M., Griffin, S.A., MacPherson, F., and Lever, A.F. Brief angiotensin converting enzyme inhibitor
treatment in young spontaneously hypertensive rats reduces blood pressure long-term. Hypertension 16, 603–614 (1990).
-
Morton, J.J., Beattie, E.C., and MacPherson, F. Angiotensin II receptor antagonist losartan has persistent effects on blood
pressure in the young spontaneously hypertensive rat: Lack of relation to vascular structure. J. Vasc. Res. 29, 264–269 (1992).
-
Paull, J.R.A. and Widdop, R.E. Persistent cardiovascular effects of chonic renin-angiotensin system inhibition following withdrawal
in adult spontaneously hypertensive rats. J. Hypertension 19, 1393–1402 (2001).
- ↵
Bergström, G., Johansson, I., Wickman, A., Gan, L., and Thorup, C. Brief treatment in young spontaneously hypertensive rats
abates long-term blood pressure elevation by effects on renal vascular structure. J. Hypertension 20, 1413–1421 (2002).
- ↵
Hu, Z.W., Shi, X.Y., Okazaki, M., and Hoffman, B.B. Angiotensin II induces transcription and expression of alpha-1 adrenergic
receptors in vascular smooth muscle cells. Am. J. Physiol. 268, H1006–H1014 (1995). First article to show Ang II-induced α1-adrenoceptor expression, both mRNA and protein, in isolated rat aorta smooth muscle cells.
- ↵
Xin, X., Yang, N., Eckhart, A.D., and Faber, J.E. α1D-Adrenergic receptors and mitogen-activated protein kinase mediate increased protein synthesis by arterial smooth muscle.
Mol. Pharmacol. 51, 764–775 (1997). The authors report that a1D-adrenoceptor activation is involved in vascular smooth-muscle-cell hypertrophy (a phenomenon observed
in hypertension).
- ↵
Villalobos-Molina, R. and Ibarra, M. α1-Adrenoceptors that mediate contraction in arteries of normotensive and spontaneously
hypertensive rats are of the α1D or α1A subtypes. Eur. J. Pharmacol. 298, 257–263 (1996). First comparative study of α1-adrenoceptor subtypes present in arteries of SHR and WKY rats.
-
Villalobos-Molina, R. and Ibarra, M. Vascular α1D-Adrenoceptors: are they related to hypertension? Arch. Med. Res. 30, 347–352 (1999).
- ↵
Ibarra, M., López-Guerrero, J.J., and Villalobos-Molina, R. Further evidence of the predominance of α1D-adrenoceptors in arteries
of normotensive and spontaneously hypertensive rats. Pharmacol. Rev. Comm. 10, 135–142 (1998). The authors report noradrenaline-induced hypersensitivity in those SHR arteries where α1D-adrenoceptors predominate for contraction (carotid and aorta), compared to WKY. Hypersensitivity is not observed with α1A- or α1A/D-adrenoceptor agonists.
-
Villalobos-Molina, R., López-Guerrero, J.J., and Ibarra, M. Functional evidence of α1D-adrenoceptors in the vasculature of
young and adult spontaneously hypertensive rats. Br. J. Pharmacol. 126, 1534–1536 (1999). This article reports the presence of α1D-adrenoceptors in pre-hypertensive SHR, not observed in young normotensive WKY, and suggests their role in the genesis and/or
maintenance of high blood pressure.
-
Guimaraes, S. and Moura, D. Vascular adrenoceptors: An update. Pharmacol. Rev. 53, 319–356 (2001).
- ↵
García-Sáinz, J.A. and Villalobos-Molina, R. The elusive α1D-adrenergic receptor: Molecular and cellular characteristics and
integrative roles. Eur. J. Pharmacol. 500, 113–120 (2004).
- ↵
Tanoue, A., Nasa, Y., Koshimizu, T., Shinoura, H., Oshikawa, S., Kawai, T., Sunada, S., Takeo, S. and Tsujimoto, G. The α1D-adrenergic
receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 109, 765–775 (2002). The authors generated mice lacking (knockout) the α1D-adrenoceptor gene. These knockout mice are hypotensive and their α1D-adrenoceptor-mediated responses are diminished.
- ↵
Lund, A.K., Goens, M.B., Kanagy, N.L., and Walker, M.K. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated
with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Toxicol. Applied Pharmacol. 193, 177–187 (2003). The authors report that knockout of the Ahr gene is associated with hypertension: increased angiotensin II and endothelin-1,
and cardiac hypertrophy are observed. Captopril treatment for three months decreased: 1) plasma concentrations of Ang II,
2) mean arterial blood pressure (MAP), and 3) heart hypertrophy.
Maximiliano Ibarra, PhD, is a pharmacologist interested in vascular α1-adrenoceptors and their interaction with endothelial function, such as nitric
oxide synthases and cyclooxygenases during aging and hypertension. He is an Associate Professor in the Biomedicine Unit at
Facultad de Estudios Superiores-Iztacala, National Autonomous University of Mexico, in Tlalnepantla, Mexico. E-mail maxibarrab{at}correo.unam.mx; fax. (52 55) 5623-1138
Rafael Villalobos-Molina, PhD, is a biochemist interested in vascular α1-adrenoceptor pharmacology and their role in the genesis/maintenance of hypertension.
He is currently Chairman of the Biomedicine Unit at Facultad de Estudios Superiores-Iztacala, National Autonomous University
of Mexico, in Tlalnepantla, Mexico. Please send correspondence to RV-M. E-mail villalobos{at}campus.iztacala.unam.mx; fax (52 55) 5623-1138.