Rare Diseases, Orphan Drugs, and Orphaned Patients
Back Story Of The Orphan Drug Act
Ronald Reagan is remembered for many things, but few would associate his presidency with the subject of medicinals. Yet it was Reagan who signed into law the two acts that have, during the past twenty-five years, had the greatest impact on the American supply of medicines. In 1984, he signed the Drug Price Competition and Patent Restoration Act, sponsored by Henry Waxman, Democratic Representative from California, and Orrin Hatch, Republican Senator from Utah. Popularly known as the Hatch-Waxman Act, this law opened the gates for American consumers to enjoy the price advantage of generic drugs and antibiotics. It allowed the generic drug industry to develop into the major enterprise it is today, at the same time giving the Food and Drug Administration (FDA) the tools to prod that industry into improved manufacturing and quality control procedures so that American generic drug products are of equal quality to their brand name counterparts. The other major piece of drug legislation presided over by Reagan was the Orphan Drug Act, which he signed on January 4, 1983. The term “orphan drug” reflects the existence of diseases that are “orphaned” (i.e., little research or pharmaceutical study is conducted) because of their rarity. Research-intensive pharmaceutical firms tend to devote their resources to widespread conditions such as asthma, diabetes, and hypertension. Aside from these and other common ailments, however, it is estimated that there are more than 6,000 rare disorders, which collectively affect some 25 million people in this country (1). Prior to 1983, these disorders were largely neglected by the drug industry.
It is just as difficult to discover and develop a remedy for a rare disease as it is for a common affliction and just as time-consuming and costly to satisfy all the necessary requirements to gain FDA approval. If the potential market comprises only a few thousand individuals, what return on investment can the drug firm expect? It is said that “big pharma” these days is not interested in a drug with potential sales of less than 100 million dollars.
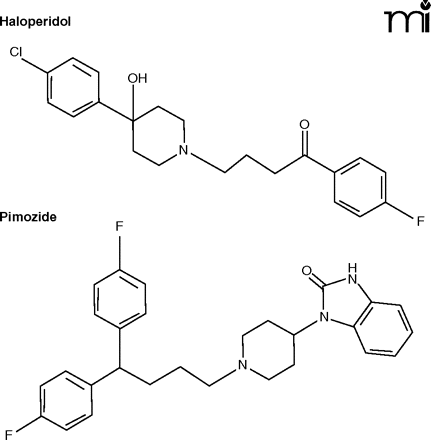
Modeling themselves after the March of Dimes and the American Cancer Society, support organizations have sprung up for many individual rare diseases, collecting funds for research and to aid patients and their families. By 1980, some of these groups had experienced first-hand the drug firms’ paucity of interest. For example, Johnson & Johnson (J&J), who make Haldol® for schizophrenia, were studying pimozide, a related compound. Although inferior to Haldol® as an antipsychotic, pimozide was found to relieve the troublesome tics of Tourette Syndrome, a rare disease thought to affect between 100,00 and 200,000 people in the US. When J&J indicated that they would have to drop pimozide, Tourette sufferers and their parents felt betrayed. Ultimately, J&J did market pimozide (Orap®), largely as a public service (2), but meanwhile the story received national media attention. As related by Ross (2), the story involved one of Henry Waxman’s constituents who contacted Waxman about his efforts to obtain pimozide from Canada for his son. The medicine was confiscated by US Customs. The Los Angeles Times carried the story and then it was picked up by Jack Klugman who based an episode of his television show “Quincy” on the dilemma of orphan drugs.
A related incident caught the attention of a US Senator. A man in Kansas whose wife had Huntington Disease––a devastating disorder that hits young people, causing loss of motor control, dementia, and early death––visited his senator, Nancy Kassebaum and explained why even if an effective treatment for his wife’s condition were discovered, it probably would never become available. Senator Kassebaum then joined Henry Waxman in sponsoring legislation to address the orphan drug issue. Lobbying by the disease-oriented groups, which later joined to form the umbrella group National Organization for Rare Disorders (NORD), together with widespread media publicity, helped in gaining Congressional passage of the Orphan Drug Act (2).
Provisions Of The Orphan Drug Act
The Act provides incentives for the testing and development of drug treatments for rare diseases (orphan drugs). An orphan drug is defined as one with efficacy against a disease affecting fewer than 200,000 people in the United States, or one that scientists and economists at the FDA determine will not be profitable for seven years after approval (3).
The incentives provided for development of orphan drugs are:
-
FDA guidance, in writing, for the clinical and nonclinical testing which must be conducted. This is particularly helpful to physician sponsors who may be excellent clinicians but have no idea as to the FDA’s specific requirements for new drugs.
-
Treatment use of investigational orphan drugs under open protocols. This helps patients all around the country who may benefit from the treatment and helps the sponsor to build up a larger clinical data base.
-
Tax credits of up to 50% of clinical testing expenses. These have also been augmented by grants to some sponsors, funded annually by the Office of Orphan Products Development (OPD).
-
A seven-year period of exclusive marketing––the greatest of the incentives, as many orphan drugs cannot be patented.
The Orphan Drug Act In Action
The Orphan Drug Act has proved a successful piece of legislation. Since its passage, 1,575 substances have received formal Orphan Designation, the prerequisite for obtaining the above incentives (4). Recently Marlene E. Haffner, the long-time Director of OPD, reflected on the Act’s accomplishments to date. She stated that since its enactment in January, 1983, some 282 orphan drugs and biologic products have been marketed, providing treatment for more than 14 million patients in the US. By contrast, between 1972 and 1982 only ten treatments for rare diseases were approved by the FDA (5).
Many rare diseases are genetically based and are often caused by the lack of a specific enzyme. One example that recently made the news is Pompe Disease, an inherited α-glucosidase deficiency affecting fewer than 10,000 people world-wide. Pompe patients cannot break down glycogen, which builds up abnormally in the muscles. Newborns with the disease generally live less than a year; if the onset is later in life, it is debilitating and life-shortening. Myozyme® was granted priority review under the Orphan Drug Act, and received FDA approval on April 28, 2006 (6). An earlier example of an enzyme-replacing orphan drug is Cerezyme®, an analog of human β-glucocerebrosidase, made by rDNA technology (7). Cerezyme® has been a life-prolonging therapy for both children and adults with Type I Gaucher disease, and, incidentally, a very profitable product for its developer (Genzyme).
The approved drug holding the record for the smallest population is pegademase (Adagen®), for combined immunodeficiency syndrome of the adenosine diaminase type, a condition affecting only fourteen patients in the US at the time of Adagen®’s approval. Indeed, approval was based on an eight-patient clinical trial (5).
Certain cancers are rare enough to fall below the prevalence limit of 200,000. Thus, a sizable number of cancer treatments have been approved as orphan drugs (Table 1⇓). Examples are imatinib (Gleevec®) for chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors, ifosfamide (Ifex®) for testicular cancer, and arsenic trioxide (Trisenox®) for acute promyelocytic leukemia (APL).
Haffner (5) states that the seven-year exclusivity provision has been credited with promoting the development of the biotech industry. Many biotech products could not be patented, as they were synthesized and published on before their medical use was established. Nonetheless, criticism has been leveled at the high price of many orphan drugs and at the fact that some have become very profitable products. There was chagrin, for example, that zidovudine (AZT), the first antiviral effective against HIV/AIDS, enjoyed orphan drug exclusivity. The irony is that at the time AZT attained orphan drug status, in 1987, there were believed to be fewer than 200,000
AIDS patients in the US. Another such product is the tyrosine kinase inhibitor Gleevec®, first approved for one rare form of leukemia but already said to enjoy blockbuster sales of over two billion dollars.
In another positive effect of the Act, some non-orphan drugs, including tretinoin and sildenafil, are being developed as treatments for rare conditions. Tretinoin, a metabolite of vitamin A, has long been available as Retin-A-Micro® for the topical treatment of acne vulgaris. It is also formulated as a cream (Renova®) for the “mitigation” of fine wrinkles of the face. Acne and facial wrinkles are both common conditions, though affecting opposite ends of the age spectrum. In 1995, tretinoin was approved as an orally administered softgel capsule (Vesanoid®) for induction of remission in the rare malignancy APL. Sildenafil (Viagra®), which enjoys widespread use for erectile dysfunction (see 8), has recently been approved at a lower dosage, marketed as Revatio®, for the treatment of the rare condition pulmonary arterial hypertension (PAH) (9). It is likely that these added indications for tretinoin and sildenafil would not have received attention were it not for the Orphan Drug Act.
An Orphan Drug Case Study
The story of zinc acetate for treatment of Wilson Disease, in which this author was closely involved, illustrates the development of an orphan drug when one clinician conceives and follows through on a novel approach to treating a rare disease (10). In the 1970s, George J. Brewer, professor of human genetics and internal medicine at University of Michigan, was attempting to treat sickle cell anemia with orally administered zinc salts. He noticed that zinc-treated patients began to show signs of copper deficiency. Because zinc administration was apparently drawing copper out of the body, he reasoned that this might be helpful for patients with Wilson Disease, a genetic error of copper metabolism in which copper builds up in the liver, brain, and other organs. Patients develop hepatic, neurologic, or psychiatric symptoms. Untreated, the disease progresses to cirrhosis and CNS damage, followed by death within a few years after onset of symptoms. The prevalence of Wilson Disease is estimated at 1 in 30,000; thus, there may be fewer than 10,000 patients in the US.
The main therapy for Wilson Disease at the time was the chelating agent penicillamine, which is an effective “decoppering” agent but can cause serious hematologic side effects and is unsafe during pregnancy. Brewer learned that a Dutch neurologist, T.O. Hoogenraad, had found zinc sulfate to be effective, but that gastric irritation precluded its long term use. Brewer chose to work with zinc acetate, an essentially neutral salt. Tablets were made for him by the hospital’s pharmacy, and over time, he enrolled several dozen patients in a long-term study.
I met Brewer at a symposium on orphan drugs in 1984, about a year after the Orphan Drug Act went into effect. Impressed by his presentation, I suggested to him that Lemmon Company, my employer, could easily manufacture zinc acetate capsules for him, as we were already making zinc sulfate capsules for some distributors of nutritional supplements. He kept the offer in mind, but contacted us only some time later, when it became clear that he and his academic colleagues could not possibly deal with the complicated requirements for the contents of a New Drug Application (NDA).
Lemmon’s first activities, upon reaching an agreement with Dr. Brewer and assuming sponsorship of the drug, were to manufacture a supply of capsules for use in the ongoing clinical trial, and to file an orphan drug application with OPD. Such an application is the proverbial “piece of cake” in contrast to an Investigational New Drug Application (IND) or NDA. In November 1985, OPD granted orphan drug status to zinc acetate for treatment of Wilson Disease.
Brewer and his associates conducted a remarkably comprehensive clinical study. They proved zinc acetate’s effectiveness in controlling Wilson Disease both subjectively and by a battery of objective tests. The latter included tests of speech and neurologic function, liver function tests, and markers of copper metabolism such as 64Cu balance studies, assays for measuring copper amounts in liver and in urinary excretion, and non-ceruloplasmin-bound (i.e., free) plasma copper levels (Box 1). The effectiveness of treatment with zinc acetate was shown to last year after year; side effects were limited to gastric irritation, which was easily controlled, and elevation of serum amylase and lipase concentration. These increased enzymatic concentrations usually returned to normal while therapy continued but were investigated in depth to make sure they did not signal pancreatic damage or other serious effects.
Binding Copper in the Plasma
Ceruloplasmin, a glycoprotein, is the principal copper transport protein, accounting for 90–95% of the circulating copper in normal mammals. Thus, normally the copper content of ceruloplasmin-free plasma is very low. Elevated concentrations of free copper not bound to ceruloplasmin would signal that Wilson Disease is not under control.
Proving safety and effectiveness was only part of Brewer’s research program. His group investigated zinc’s mode of action, validated the use of 64Cu in copper-balance studies and monitored the treatment’s efficacy via urine and plasma Cu assays (as stated above), looked for potential drug interactions, studied preventive zinc treatment of presymptomatic siblings of Wilson Disease patients, and discovered a linkage between Wilson Disease and a gene on chromosome 13. Note that all this was accomplished without the resources of big pharma.
Then came the time to prepare the NDA. The firm, Lemmon, sent a consultant out to Ann Arbor to assist Brewer in tabulating his vast amount of clinical and biochemical data in the form acceptable to FDA reviewers. Just then the European chemical firm which had been supplying bulk zinc acetate for seven years chose to discontinue manufacturing the salt. This sent Lemmon’s materials management people scurrying to find a new source operating under Good Manufacturing Practices (GMP). Fortunately, such a plant was found, only ninety miles away in Pennsylvania. Finally, the process of assembling all the documentation, circulating it for in-house review, auditing, paginating, and photocopying was completed, and the NDA was submitted in June 1994—eight-and-a-half years after receiving orphan drug designation and seventeen years after Brewer began his studies.
The NDA review for this orphan drug was, in general, as rigorous as that for any drug of great commercial potential. Thus, pre-approval inspections were conducted at the clinical trial site (University of Michigan), at the chemical plant where the active ingredient was to be manufactured, and at the sponsor’s facility where the drug product was made. The FDA also had the Lemmon perform a bioavailability study of zinc plasma levels following oral administration of single and multiple doses of the capsules, even though the site of zinc’s action is at the cells of the intestinal wall. An advisory committee meeting was convened as well. On the other hand, the Agency allowed Hoogenraad’s study to be used as a second clinical trial, and historical data on untreated patients to substitute for a placebo control. On balance, the firm felt that FDA’s helpful actions outweighed any perceived difficulties or delays (10).
Orphaned Patients
Would anyone imagine that there could be a shortage of thiamine (Vitamin B1) in this country? Yet, an impassioned letter from a physician in Michigan reports that there recently was a three-month nationwide shortage of intravenous (iv) formulations of thiamine, the second in the past three years. The product is made by only one firm, so any problem at their plant cuts off the entire country’s supply. This physician’s letter lists several B1-deficiency disorders that may become medical emergencies requiring iv or intramuscular (im) treatment (11).
Similarly, it was reported that Pfizer, the sole supplier of spectinomycin (Trobicin®), is discontinuing US distribution of the product. Although spectinomycin is a rarely prescribed antibiotic, it is still useful for treating gonorrhea patients who cannot be given fluoroquinolones or third-generation cephalosporins. It is also the preferred treatment for gonorrhea in pregnant patients with cephalosporin allergy, because fluoroquinolones are contraindicated during pregnancy. Finally, it is used for gonorrhea in homosexual men with penicillin/ cephalosporin allergies (12).
An earlier Reflections piece in this journal noted that an American soldier or tourist returning from abroad may occasionally suffer a fulminant case of malaria requiring immediate parenteral treatment, the preferred drugs for this condition being iv forms of quinine or artemisinin (13). Neither of these is FDA-approved, probably because the rare need does not warrant the effort and expense of an NDA.
These three examples—probably the tip of a sizable iceberg—point to the existence of a class of patients who may be termed “orphaned patients.” The medicine they need does not have to be newly discovered or developed; it is known, but market forces have caused it to be unavailable. The medical and pharmacy professions need to determine the extent of this problem and make recommendations for its alleviation.
Another aspect of this problem (i.e., lack of gainful profit) exists in underdeveloped parts of the world, and affects huge numbers of people. One example is malaria, for which the recommended artemisinin-based therapy consists of artesunate together with amodiaquine or mefloquine. Separately, the sick patient must swallow seven or eight tablets a day, making adherence to the regimen difficult. A non-profit collaboration, the Drugs for Neglected Diseases Initiative (DNDI), was founded in 2003 by Doctors Without Borders, the World Health Organization (WHO), and several national research institutions. DNDI has now announced two fixed-dose combinations, artesunate/amodiaquine (to be manufactured and distributed by Sanofi-Aventis, Paris) and artesunate/mefloquine (to be manufactured and distributed by Far-Manguinhos, a pharmaceutical laboratory owned by the government of Brazil), which will supply the full dosage in only two tablets per day. DNDI has arranged for manufacture and distribution of the two combinations, free of patent or exclusivity constraints (14).
Conclusion
The Orphan Drug Act has achieved its objectives. Patients suffering from rare diseases are receiving treatments that might otherwise never have been developed. The orphan drugs approved to date range chemically from simple salts (e.g., zinc acetate), to complex multicyclic synthetics (e.g., imatinib), to biotechnologically engineered enzymes (e.g., Cerezyme®). As further evidence of its success, the Orphan Drug Act has served as a model for orphan drug laws enacted in the European Union, Japan, and Australia (5).
Of equal importance, attention has now been focused on rare conditions. Orphan drug development is no longer looked on as a charitable act, but as an avenue to basic biomedical knowledge and often a source of profit. Two recent examples illustrate this change of viewpoint. PAH, mentioned above in connection with sildenafil, is said to affect some 50,000 people in the US and 200,000 world-wide; hardly, it would seem, a target for intensive drug R&D. Yet, the status of clinical testing of three investigational drugs for PAH was reported in two finance-oriented publications (15, 16). CML, mentioned in regard to imatinib, hits fewer than 5,000 patients per year in the US. Clinical trials on two newer kinase inhibitors were reported in a recent issue of the New England Journal of Medicine, prompting an editorialist to ask why two major drug firms would be interested in so rare a disease. The answer, in his view, is not only the dollar value of the CML market, but also the fact that the drugs can be tested in a “small, molecularly defined population...with a high probability of having a response to therapy” (17). The editorialist feels also that the trials in question are early applications of genomic medicine (17).
In a highly significant development, the National Institutes of Health (NIH) has just announced that its Rare Diseases Clinical Research Network is ready to launch studies on a variety of rare disorders, among which are Prader-Willi syndrome, myelodysplastic syndrome, certain lung diseases, several vasculitides, urea cycle disorders, and rare pediatric liver diseases. Establishment and funding of this network resulted from two laws signed by President Bush in 2002: the Rare Diseases Act and the Rare Diseases Orphan Product Development Act. These Acts increased rare disease research funding for both the NIH and OPD. During the past three years the network has established protocols, recruited investigators throughout the country, arranged for drug companies to provide currently available drugs for study in new indications, and set up a data and technology coordinating center. Patients are now being enrolled for the first dozen-plus studies (18).
As this article opened with mention of a former President, it may be appropriate to close with a reference to Congress. The Orphan Drug Act, as well as the equally successful Hatch-Waxman Act, resulted from productive collaboration and compromise between legislators of widely opposing political views. Today’s Congress would do well to take note and adopt a similar strategy.
Numbers of Orphan Drugs Approved by the FDA for Orphan Diseases
- © American Society for Pharmacology and Experimental Theraputics 2006
References

Stanley Scheindlin, DSc, RPh, holds graduate degrees in pharmaceutical chemistry and worked in drug product development and regulatory affairs. Now retired, he is a part-time consultant and writes freelance articles for pharmacy-related specialty publications.