Emerging concepts from the recent literature
hOAT2 is a Bi-Directional cGMP Transporter
Whereas the regulation of cyclic GMP (cGMP), a cellular second messenger that mediates both intracellular and extracellular responses to the environment, has been investigated and validated as a useful therapeutic approach (e.g., Viagra), the mechanisms involved in cellular uptake of cGMP and guanine nucleotides remain poorly understood. An article by Cropp et al., a Fast Forward publication in Mol. Pharmacol., tested whether guanine nucleotides could interact with the human organic anion transporter 2 (hOAT2, SLC22A7). The authors identified hOAT2 as a bi-directional cGMP transporter, bringing extracellular cGMP into the cell under basal conditions but effluxing cGMP when cells are treated with sodium nitroprusside and 3-isobutyl-1-methylxanthine (IBMX). Also, they showed that a previously identified splice variant failed to localize to the plasma membrane and thus lacked transport activity. Because hOAT2 is highly expressed in numerous tissues, hOAT2 may work along with ABCC transporter isoforms, guanylate cyclase, and phosphodiesterase to regulate intracellular cGMP concentrations. Thus, hOAT2 may represent a new target for regulating cGMP concentrations. [Cropp, C.D., Komori, T., Shima, J.E., Urban, T.J., Yee, S.W., More ,S., and Giacomini, K.M. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol. Pharmacol. Epub ahead of print; doi: 10.1124/mol.107.043117 (2008).]
—PM Gerk, Virginia Commonwealth Univ.
A Possible New Drug for Treating the CF Patient
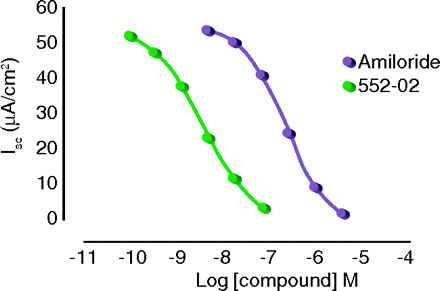
Airway dehydration is a major manifestation of cystic fibrosis (CF), resulting in thick, tenacious mucus secretion that impedes efficient gas exchange. Aerosolized amiloride has been used to block hyperactive Na+ reabsorption in the CF lung, but clinical outcomes have been less than optimal. In the Fast Forward section of J. Pharmacol. Exp. Ther., Hirsh et al. report the development of a promising, long-acting amiloride analog, 552-02, which was specifically designed for use in CF. As compared to amiloride, its potency is two orders of magnitude greater; its binding is far less reversible; its systemic absorption is much slower than that of the parent compound; and it is compatible with current hypertonic saline therapy. Most notably, 552-02 produced a sustained increase in mucociliary clearance in sheep, an effect not observed with amiloride. Thus, 552-02, by blocking airway epithelial Na+ channels, represents a potentially exciting advance in the treatment of the CF patient. [Hirsh, A.J., Zang, J., Zamurs, A. et al. Pharmacological properties of 552-02, a novel epithelial sodium channel blocker with potential clinical efficacy for cystic fibrosis lung disease. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.107.130443 (2008).]
—DJ Benos, University of Alabama-Birmingham
Pain: Cannabinoid and Chemokine Interconnections
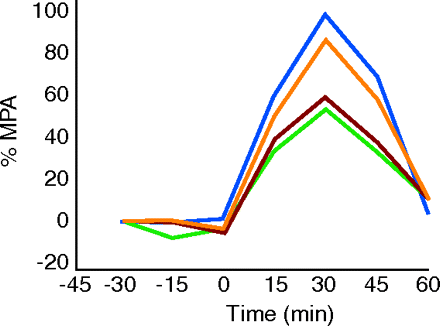
Cannabinoids (CBs) have emerged as a promising class of drugs to treat pain, but the precise mechanisms and systems involved in mediating CB-induced analgesia remain unknown. Chemokines might modulate CB-mediated pain management, and indeed, in a J. Pharmacol Exp. Ther. article published online (February 15), Benamar et al. examined whether chemokines could alter CB-induced analgesia using a rodent model. Administration of the CB receptor agonist (+)-WIN 55,212-2 into the periaqueductal gray (PAG) of the midbrain resulted in a dose-dependent analgesic response that was blocked by the chemokine SDF-1α/CXCL12, when administered in the same brain region. The effect of SDF-1α/CXCL12 was mediated through the chemokine receptor CXCR4, given that a selective antagonist for this receptor reversed the effects of SDF- 1α/CXCL12. This is the first study to report that activation of the chemokine receptor CXCR4 in the PAG can interfere with CB-induced analgesia. [Benamar, K., Geller, E.B., and Adler, M.W. First in vivo evidence for a functional interaction between chemokine and cannabinoid systems in the brain. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.107.135053 (2008).]
—TV Khroyan, SRI International
Antipsychotic-Mediated Neuroplasticity
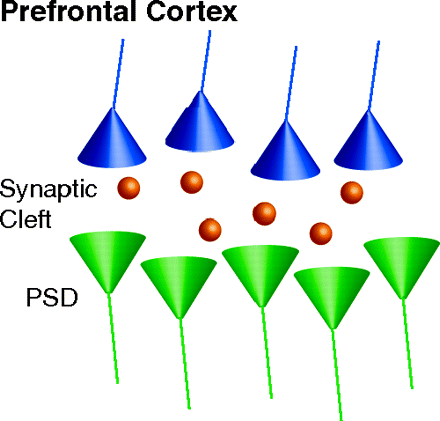
Abnormal communication between brain dopaminergic and glutamatergic systems could be critical for eliciting psychosis. Antipsychotics targeting monoaminergic systems are used to treat schizophrenia. How antipsychotic treatments affect glutamatergic synapse remains unexplored. In the Fast Forward edition of Mol. Pharmacol., Fumagalli et al. investigate the dynamic regulation of glutamatergic postsynaptic activity in the prefrontal cortex induced by chronic treatment with the antipsychotics haloperidol and olanzapine. Analysis of neuronal subcellular fractions revealed that haloperidol, but not olanzapine, causes multilevel adaptations at the glutamate synapse involving reduced amounts of glutamate NMDA receptor subunits NR1 and NR2A and scaffolding protein PSD-95. In addition, deficient trafficking of GluR1 to the membrane and changes in the total and phosphorylated levels of CaMKII at synaptic sites were observed. This study provides a novel perspective for understanding chronic antipsychoticinduced neuroplasticity that could be critical for beneficial or side effects of different classes of antipsychotics. [Fumagalli, F., Frasca, A., Racagni, G., and Riva, M.A. Dynamic regulation of glutamatergic post-synaptic activity in rat prefrontal cortex by repeated administration of antipsychotic drugs. Mol. Pharmacol. Epub ahead of print; doi:10.1124/mol.107.043786 (2008).]
—RR Gainetdinov, Duke University
- © American Society for Pharmacology and Experimental Theraputics 2008



