
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Let’s Go Rafting: Ligand Functional Selectivity May Depend on Membrane Structure
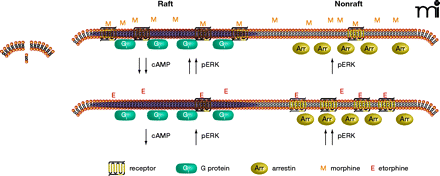
Functional selectivity of μ-opiate receptor ligands and membrane raft domains. Upon binding of morphine (top panel, left), the receptor conformation has a greater affinity for Gi, which is localized to the raft domain. Signaling through Gi inhibits adenylyl cyclase to decrease cyclic AMP and leads to increased phosporylation of ERK. The morphine-activated receptor has a lower affinity for β-arrestin––localized to nonraft domains (top panel, right)––which leads to ERK phosphorylation. The etorphine-bound receptor (bottom panel, right), favors coupling to β-arrestin, leading to greater ERK activation and relatively lesser inhibition of adenylyl cyclase. Arr, β-arrestin; cAMP, cyclic adenosine 3′,5′-monophosphate; E, etorphine; ERK, extracellular signal–regulated protein kinase; Gi, inhibitory G protein; M, morphine.


