
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Fragment-based ligand discovery
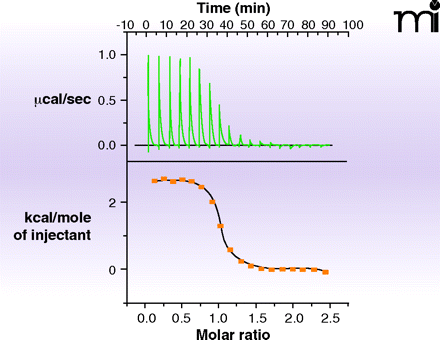
Figure 6.
An example of isothermal titration calorimetry (ITC). The heat evolved or absorbed as a compound binds to a protein is a calorimetric property. ITC is limited by high solubility requirements for ligand and protein and by the amount of heat generated, but typically can measure KD values in the range of 10nM to 500μM. Shown in the data here is the titration of the tripeptide K-cyclohexylalanine-K to the protein OppA. The upper curve is the direct measurement of heat generated as ligand is added to protein; the bottom curve plots these values to provide a KD, which combined with the total heat generated (area under the curve) allows enthalpy and entropy to be estimated.


