Emerging concepts from the recent literature
PS: We’re not flipping out!
Externalization of phosphatidylserine (PS) is a well-known part of apoptosis, although the mechanism has been poorly understood. Previously, it has been thought the PS in the outer leaflet of the plasma membrane was solely transferred from the inner leaflet by “scramblases.” Recent work by Mirnikjoo et al., however, clarifies the role of PS display in the context of membrane damage repair. This context comprises two observations that are related to an increase in intracellular calcium concentrations (i.e., [Ca2+]i). The first of these is that membrane damage (e.g., a membrane leak) will result in an increase in [Ca2+]i, where-upon lysosomes fuse with the plasma membrane to repair the damage. The second observation is that PS display can also be induced transiently with increased [Ca2+]i—even in the absence of apoptotic stimuli. By specifically labeling lysosomes and PS with fluorescent dyes, Mirnikjoo et al. now demonstrate that induced lysosome-plasma membrane fusion is invariably linked to increased PS in the plasma membrane. Furthermore, blocking lysosome fusion prevents PS display, without inhibiting other aspects of apoptosis. Intriguingly, it thus appears the PS displayed on the plasma membrane originates, at least in part, from the lysosomal membrane. This work may have important implications for apoptotic pathways that affect cancer therapy. PS display is a cue for treated cancer cells to be engulfed by phagocytes. The authors suggest that inhibitors of lysosome–plasma membrane fusion might prevent PS externalization during apoptotic death, thereby promoting an immune response to cancer cells. Indeed, early clinical studies show that lysomotrophic agents, when added to traditional chemotherapies, can improve clinical outcomes. [Mirnikjoo, B., Balasubramanian, K., and Schroit, A.J. Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis. J. Biol. Chem. 284, 22512–22516 (2009).] –CD Zellefrow, U Pittsburgh
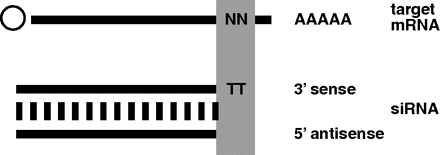
Chemotherapeutic short interfering RNAs: It’s all in the “wrapping”
Short interfering RNAs (siRNAs) have been developed into a robust and effective gene-silencing strategy that has recently been investigated as a potential therapeutic approach in a variety of diseases, including cancer. The therapeutic use of siRNAs, however, has been limited by their biological instability, which contributes to problems of biocompatibility in the development of suitable delivery systems. In a recent report from Cancer Research, Yagi et al. propose a nano-particle system termed the “wrapsome,” which contains an siRNA/cationic lipofection complex that is fully enveloped by a neutral lipid bilayer and hydrophilic polymers. Using this wrapsome delivery system, the authors show they can increase the in vivo half-life of an siRNA from 4 minutes to 17.6 hours and abrogate the susceptibility of the siRNA to RNase. Using LL/2 and PC-3 tumor xenografts, they demonstrate an increased localization and accumulation of Cy5-labeled siRNAs that persist after 11 days; without use of the wrapsome technology, the same siRNA species are completely abolished within 5 days. Upon treatment of the xenografts with an siRNA against Krüppel-like zinc finger transcription factor 5 (KLF5), an essential component of angiogenesis, they demonstrated a significant decrease in tumor volume as well as an increase in survival, with no signs of significant acute or long-term toxicity. This report provides an interesting new insight into the use of nanoparticles as a specific, nontoxic delivery system of therapeutic siRNAs. The promise of the wrapsome technology may silence some of the occasional cynicism that has been associated with siRNA pharmaceutical challenges. [Yagi, N., Manabe, I., Tottori, T. et al. A nanoparticle system specifically designed to deliver short interfering RNA inhibits tumor growth in vivo. Cancer Res. 69, 6531–6538 (2009).] –CA Kitchens, U Pittsburgh
- Copyright © 2009



