 |
 |

Effectiveness and Costs of Omeprazole vs Ranitidine for Treatment of Symptomatic Gastroesophageal Reflux Disease in Primary Care Clinics in West Virginia
Barbara Kaplan-Machlis, PharmD;
Gerard E. Spiegler, MS;
Marc W. Zodet, MS;
Dennis A. Revicki, PhD
Arch Fam Med. 2000;9:624-630.
ABSTRACT
 |  |
Objective To compare clinical, health-related quality of life (HRQL), and medical cost outcomes in patients with symptomatic gastroesophageal reflux disease (GERD) receiving omeprazole sodium or ranitidine hydrochloride treatment.
Methods A multicenter, randomized, open-label, medical effectiveness trial conducted in 5 university-based family medicine clinics. Two hundred sixty-eight patients with GERD were recruited and randomly assigned to omeprazole sodium, 20 mg once daily, or ranitidine hydrochloride, 150 mg twice daily, for up to 6 months. Main outcome assessments included the Gastrointestinal Symptom Rating Scale (GSRS) Reflux score, Psychological General Well-Being Index, and Short-Form–36 Health Survey administered at baseline and 2, 4, 12, and 24 weeks. Medical resource use and cost data were collected.
Results More omeprazole-treated patients reported improved heartburn resolution at 2 weeks (49.0% vs 33.3%; P=.007) and 4 weeks (58.6% vs 35.0%; P<.001) compared with ranitidine-treated patients. The GSRS Reflux scores across 3 months showed overall differences between omeprazole (mean, 2.67) and ranitidine (mean, 2.95) groups (P=.04). Mean total 6-month medical costs were $915 lower ($8371 vs $9286; P=.64), and no difference in mean outpatient medical costs ($1198 vs $1158; P=.76) were observed in the omeprazole group compared with the ranitidine group. A post hoc secondary analysis showed that, at 12 and 24 weeks, patients treated with omeprazole for 8 weeks or more reported greater heartburn resolution (ie, 24 [43%] of 56 patients at both intervals) than patients treated with ranitidine for 8 weeks or more (12 [24%] and 13 [26%] of 50 patients, respectively; P=.001).
Conclusions Ranitidine and omeprazole were both effective at improving heartburn symptoms; however, omeprazole provided greater resolution of heartburn symptoms at 2 and 4 weeks. Despite omeprazole's higher acquisition cost, there were no significant differences in total or outpatient costs between groups.
INTRODUCTION
GASTROESOPHAGEAL reflux disease (GERD) is a common disorder that results from inappropriate reflux of gastric contents from the stomach back into the esophagus. It is prevalent in the primary care and general populations.1-2 Most patients who present to and are treated by primary care physicians have mild to moderate symptoms (eg, heartburn, acid regurgitation) and relatively uncomplicated disease. The frequency and severity of heartburn and other GERD-related symptoms are associated with impairment in patient functioning and well-being or health-related quality of life (HRQL).3-4
The primary focus of medical treatment for GERD is to reduce the acid content of reflux. Treatment goals are to relieve pain and GERD-related symptoms; to decrease frequency and duration of reflux; to heal erosions, if present; and to prevent recurrence.5-6 Pharmacological treatment with acid suppressant agents (eg, histamine2 receptor antagonists [H2RAs] and proton pump inhibitors [PPIs]) is the mainstay of therapy.
Ranitidine hydrochloride, an H2RA,7-10 and omeprazole sodium, a PPI,11-15 have demonstrated effectiveness in control of heartburn in patients with symptomatic GERD and in improvement of HRQL, compared with placebo. Several clinical trials have shown that compared with ranitidine, omeprazole provides greater improvement in psychological well-being and HRQL outcomes in patients with symptomatic GERD.14-15 Several clinical trials have compared H2RAs and PPIs1, 13, 15-17; most of these studies were in patients with endoscopically confirmed esophagitis. The control of heartburn symptoms was better in patients with GERD who were treated with omeprazole; however, it is not clear whether these findings can be extrapolated to primary care practice.
Few cost-effectiveness analyses have been completed comparing ranitidine vs omeprazole for the treatment of symptomatic GERD.18-19 Although the medication costs of the H2RAs are clearly lower than those of omeprazole, differences in the use of GERD-related medical services and patient outcomes may favor omeprazole.18-19
This naturalistic clinical trial was conducted to evaluate the clinical, HRQL, and medical cost outcomes of omeprazole vs ranitidine treatment for patients with clinically diagnosed, symptomatic GERD. The study was performed in clinics affiliated with a university-based department of family medicine and located in a catchment area that included a large proportion of rural primary care patients. Naturalistic or pragmatic clinical trials are best suited to answer research questions regarding treatment effectiveness and costs in usual practice settings, but cannot address questions about treatment efficacy.20
PATIENTS AND METHODS
A prospective, multicenter, open-label randomized clinical trial was conducted to compare the clinical, HRQL, and medical cost outcomes of patients with symptomatic GERD initially treated with omeprazole or ranitidine. Patients were enrolled during a 24-month period from 5 clinics affiliated with a university-based department of family medicine. Physicians identified patients with a clinical diagnosis of GERD who, in the physician's judgment, needed to start medication treatment. Diagnosis of GERD was based on frequency of heartburn and/or acid regurgitation,5-6,21 despite nonprescription treatment for 2 weeks or more. Study patients were aged 18 years or older, with no PPI or H2RA treatment in the previous 30 days or contraindications to the study treatments, were not pregnant or lactating, and had no clinical evidence of renal or hepatic insufficiency. Eligible patients were randomly assigned to unblinded omeprazole or ranitidine therapy, and all subsequent medical care (eg, dose changes, diagnostic tests) was managed by their primary care physicians as per usual care. Before the start of the study, the university institutional review board approved the research protocol, and all study participants provided written informed consent.
TREATMENT REGIMEN
Computerized randomization was used, and the study coordinator prepared labeled medication vials before study commencement that were placed in sealed envelopes and stored at each study site. Once study patients were identified and informed consent was complete, patients were given a study packet that contained a sealed envelope with the 30-day supply of their assigned drug, an advertisement regarding the study goals and incentives, response scales to use during interviews, and a schedule of follow-up calls. The enrolling prescriber was aware of the assigned treatment before completion of the initial visit. Patients were assigned randomly to treatment with omeprazole sodium, 20 mg once daily (n=138), or ranitidine hydrochloride, 150 mg twice daily (n=130). All subsequent prescriptions were filled through the patients' usual pharmacy under their usual health insurance coverage. All patients were observed for 6 months regardless of duration of study drug treatment, unless they withdrew consent to participate in the clinical trial. Patients remained under the care of their primary care physician throughout the study, and physicians were free to prescribe treatment as clinically necessary, including any modification to GERD medication treatment. There were no mandated, protocol-related visits. Physicians were free to schedule follow-up visits, specialist referrals, and diagnostic procedures (eg, endoscopy) according to their usual clinical practice.
PATIENT OUTCOME ASSESSMENTS
Baseline and follow-up patient demographic, symptom, and HRQL assessments were administered by telephone interview by a trained research coordinator (G.E.S.) unaware of treatment assignment. Data on gastrointestinal tract symptoms, HRQL, and medical resource use were collected at 2, 4, 12, and 24 weeks. Patients underwent follow-up assessment regardless of whether they continued study medication treatment.
CLINICAL SYMPTOM MEASURES
The Gastrointestinal Symptom Rating Scale (GSRS) was used to assess patient report of gastrointestinal tract symptoms. The GSRS consists of 15 items evaluating common gastrointestinal tract symptoms.22 Respondents rate each question using a 7-point Likert scale ranging from "no discomfort at all" to "very severe discomfort," with a higher score indicating more severe discomfort with symptoms. Scores for total GSRS and Reflux (heartburn, acid regurgitation), Indigestion, Abdominal pain, Diarrhea, and Constipation subscales are determined by averaging related items. The GSRS has good reliability and validity.22-23
The primary clinical end point was defined as heartburn resolution based on the GSRS. Resolution of heartburn symptoms was classified as patient response to the GSRS heartburn item indicating no discomfort related to heartburn during the past week. A secondary clinical end point was the GSRS Reflux subscale score.
HRQL MEASURES
Health-related quality of life was assessed using the Psychological General Well-Being (PGWB) Index24 and Short-Form–36 (SF-36) Health Survey.25 The PGWB is a generic instrument that consists of 22 items measuring psychological well-being and distress.24 Respondents rate each question using a 6-point scale, with a higher score indicating better health status and psychological well-being. The PGWB has excellent reliability and validity, and has been used in previous GERD studies.3, 26-27
The SF-36 is a generic health status measure designed to assess functioning and well-being.25 The SF-36 contains 8 domain scales, and Mental Component Summary (MCS) and Physical Component Summary (PCS) scores are constructed.28 Extensive evidence supports the reliability and validity of the SF-36,25, 28 and this measurement tool has been used in previous clinical trials comparing treatments for GERD.3, 10, 27 For this study, we report data only on the PCS and MCS scores.
MEDICAL SERVICE USE AND COSTS
The patients were asked to provide information on all outpatient physician visits, specialist visits, diagnostic procedures (eg, endoscopy), emergency department visits, hospitalizations, and use of prescription medications during the 6-month follow-up period. We verified patient reported data by reviews of clinic and hospital records and computerized administrative records. For hospitalizations, we collected use and cost information on primary care and specialty physician inpatient services. No data were collected on the use and cost of over-the-counter medication for heartburn, since we did not think we could collect accurate quantitative data on the use of these medications from patient interviews.
We estimated the costs of outpatient physician and diagnostic services and inpatient hospital services based on the actual charges from university billing and accounting system records. We estimated the costs of all medications using Red Book average wholesale prices.29 Medical costs for all patients were aggregated into total, outpatient, inpatient, and medication cost categories. Since we were able to gather information on all medical resource use and costs, no imputation of medical costs was needed. All costs are given in 1998 dollars.
STATISTICAL ANALYSIS
Baseline demographic, clinical, and HRQL characteristics for the 2 treatment groups were compared by means of  2 tests for categorical variables and t tests for continuous variables. We also compared baseline characteristics of patients with (n=251) and without (n=17) follow-up data by means of 2 tests for categorical variables and t tests for continuous variables. We also compared baseline characteristics of patients with (n=251) and without (n=17) follow-up data by means of  2 and t tests. 2 and t tests.
An intention-to-treat approach was taken for all patient outcome and medical cost comparisons. For the analysis of heartburn resolution, patients with missing data or who withdrew from the study were counted as failures (ie, patients continued to experience heartburn symptoms). Resolution of heartburn by treatment group at 2, 4, 12, and 24 weeks was evaluated by means of logistic regression analysis, controlling for baseline GSRS Reflux scores. Adjusted treatment comparisons of GSRS Reflux and HRQL scores were conducted using mixed-model analysis of variance (ANOVA) techniques, with a compound symmetry covariance matrix. The ANOVAs included terms for treatment group (omeprazole vs ranitidine), assessment (0, 4, 12, and 24 weeks), clinic location (urban vs rural), interactions of treatment group by clinic location, and treatment group by assessment.
Medical cost data were logarithm-transformed before data analysis. For the medical cost outcomes, we used ordinary least squares regression analysis to compare mean costs by treatment group. These regression analyses included age, sex, and clinic location; comorbidity status (ie, presence of 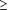 1 chronic medical condition); and baseline PCS scores. 1 chronic medical condition); and baseline PCS scores.
A post hoc secondary analysis compared treatment group differences in heartburn resolution, GSRS Reflux scores, PGWB total scores, and total and outpatient medical costs for the following 4 treatment duration groups: (1) omeprazole treatment for less than 8 weeks (n=71); (2) omeprazole treatment for at least 8 weeks (n=56); (3) ranitidine treatment for less than 8 weeks (n=69); and (4) ranitidine treatment for at least 8 weeks (n=50). The 8-week time interval was selected because many patients covered by Medicaid were shifted to other medications between 8 and 12 weeks under state policy. We used  2 tests to compare resolution of heartburn during 24 weeks. Mixed-model ANOVA was used to compare differences in mean Reflux and PGWB total scores. Ordinary least squares regression analysis, including age, sex, clinic location, comorbidity status, and baseline PCS scores, was used to compare mean total and outpatient medical costs by treatment duration group. A P value of .05 was used for all statistical comparisons. No adjustments were made for multiple comparisons; interpretation of statistical significance took into consideration the number of tests. 2 tests to compare resolution of heartburn during 24 weeks. Mixed-model ANOVA was used to compare differences in mean Reflux and PGWB total scores. Ordinary least squares regression analysis, including age, sex, clinic location, comorbidity status, and baseline PCS scores, was used to compare mean total and outpatient medical costs by treatment duration group. A P value of .05 was used for all statistical comparisons. No adjustments were made for multiple comparisons; interpretation of statistical significance took into consideration the number of tests.
RESULTS
During a 24-month period, 268 patients entered the study. One hundred thirty-eight patients were randomized to omeprazole treatment; 130, ranitidine treatment. Seventeen patients (6.3%) discontinued the clinical trial without any follow-up assessments, including 8 (5.8%) in the omeprazole group and 9 (6.9%) in the ranitidine group (P=.71). Study dropouts were not significantly different from patients with 1 or more follow-up assessments on baseline demographic, clinical, or HRQL measures (data not shown).
There were no statistically significant differences between the omeprazole and ranitidine treatment groups on demographic or baseline GSRS or HRQL scores (Table 1). The ranitidine group reported baseline GSRS Reflux scores that were slightly higher than those of the omeprazole group (P=.06).
|
|

|
|
Table 1. Baseline Demographic, Clinical Symptom, and HRQL Variables by Treatment Group*
|
|
|
RESOLUTION OF HEARTBURN SYMPTOMS
Figure 1 summarizes the percentage of patients with resolution of heartburn symptoms by treatment group. After 2 weeks, 49.0% of omeprazole- and 33.3% of ranitidine-treated patients reported no discomfort with heartburn symptoms (P=.007). At 4 weeks, 58.6% of omeprazole- and 35.0% of ranitidine-treated patients reported no discomfort with heartburn symptoms (P<.001). There was attenuation in heartburn resolution status in both groups, and no significant treatment group differences in heartburn resolution were observed at 12 (P=.14) or 24 weeks (P=.18).
|
|

|
|
Figure 1. Complete resolution of heartburn symptoms by treatment with omeprazole sodium or ranitidine hydrochloride.
|
|
|
GERD SYMPTOMS
Figure 2 shows mean GSRS Reflux scores by treatment group over time. The mixed-model ANOVA indicated no interaction effect of treatment by assessment (P=.09) and no main effect of treatment (P=.20) during 6 months. There was no difference in adjusted mean Reflux scores during 6 months (omeprazole group, 2.68; ranitidine group, 2.85). A secondary analysis comparing only 2- and 4-week Reflux scores demonstrated a significant treatment effect (P=.005). The omeprazole group reported lower adjusted mean Reflux scores (adjusted 1-month mean score, 2.53) compared with the ranitidine group (adjusted 1-month mean score, 2.89), suggesting improvement in GERD symptoms during the initial month of therapy. When these ANOVAs were repeated restricting follow-up to 3 months, we found statistically significant treatment differences (P=.04) favoring the omeprazole group (adjusted 3-month mean score, 2.67) compared with the ranitidine group (adjusted 3-month mean score, 2.95).
|
|

|
|
Figure 2. Adjusted mean Gastrointestinal Symptom Rating Scale (GSRS) Reflux scale scores by treatment with omeprazole sodium or ranitidine hydrochloride.
|
|
|
HRQL OUTCOMES
Figure 3 summarizes the PGWB total scores by treatment group for each follow-up assessment. The mixed-model ANOVA indicated no interaction effect of treatment by assessment (P=.35) and no main effect of treatment (P=.66) during 6 months. For MCS scores, there were no significant treatment-by-assessment interactions (P=.25) or main effects of treatment (P=.69) (not shown). For PCS scores, there were no significant treatment-by-assessment interactions (P=.13) or main effects of treatment (P=.82) (data not shown).
|
|

|
|
Figure 3. Adjusted mean Psychological General Well-Being (PGWB) Index total scores by treatment with omeprazole sodium or ranitidine hydrochloride.
|
|
|
MEDICAL COSTS
Total and disaggregated medical costs are reported in Table 2. Although there was a $915 difference in total costs, the regression analysis demonstrated no statistically significant difference between the omeprazole and ranitidine groups (P=.64). Outpatient costs were comparable between both treatment groups (P=.76).
|
|

|
|
Table 2. Mean Medical Costs by Treatment Group
|
|
|
POST HOC ANALYSIS OF PATIENT OUTCOMES AND COSTS BY TREATMENT DURATION
Five patients (3 treated with omeprazole and 2 treated with ranitidine) were excluded from the post hoc analysis because of missing data regarding their supply of medication. At 1 month, the omeprazole-treated groups reported more heartburn resolution (41 [58%] of 71 patients and 34 [61%] of 56 patients) compared with the ranitidine-treated groups (24 [35%] of 69 patients and 17 [34%] of 50 patients) (P=.002; Figure 4). By week twelve, 24 (43%) of the 56 patients treated with omeprazole for at least 8 weeks reported complete heartburn resolution compared with 12 (24%) of 50 patients treated with ranitidine for at least 8 weeks, 18 (26%) of 69 patients treated with ranitidine for less than 8 weeks, and 19 (27%) of 71 patients treated with omeprazole for less than 8 weeks. The 43% (24 of 56 patients) heartburn resolution was maintained during 24 weeks of follow-up for that group. The group treated with omeprazole for less than 8 weeks showed improvements in clinical outcome during the first month, followed by decreases to the levels experienced by the ranitidine treatment groups.
|
|

|
|
Figure 4. Percentage of complete heartburn resolution by duration of treatment with omeprazole sodium or ranitidine hydrochloride.
|
|
|
The mixed-model ANOVA for mean Reflux scores demonstrated a statistically significant interaction of treatment duration group by assessment (P=.04). This interaction effect was attributable to the improvement in GSRS Reflux scores in the group treated with omeprazole for less than 8 weeks during the first month, with deterioration in Reflux scores at 12 and 24 weeks (data not shown). No statistically significant differences in mean PGWB total scores were observed among the 4 treatment duration groups during the 24-week study (data not shown).
Statistically significant differences in mean total medical costs were seen among the 4 treatment duration groups (P=.03; Table 3). The groups treated with omeprazole for less than 8 weeks and ranitidine for at least 8 weeks had the highest mean total medical costs. Mean differences in outpatient medical costs were statistically significant among the 4 treatment duration groups (P<.001). The groups treated with omeprazole and ranitidine for at least 8 weeks had comparable mean outpatient costs (P=.84). Although the group treated with omeprazole for less than 8 weeks had somewhat higher mean outpatient medical costs, there was no statistically significant difference between the groups treated with omeprazole and ranitidine for less than 8 weeks (P=.11).
|
|

|
|
Table 3. Mean Total and Outpatient Medical Costs by Treatment Duration Group
|
|
|
COMMENT
Patients treated with omeprazole were more likely to report resolution of heartburn symptoms in 4 weeks compared with patients treated with ranitidine. These advantages of omeprazole were attenuated after 3 and 6 months of follow-up. Symptoms related to GERD (ie, heartburn and acid regurgitation) also were reduced in the omeprazole group during the initial month of treatment. These findings are consistent with the clinical effectiveness findings in other clinical trials.14-15,18
No significant differences in HRQL outcomes were observed between the treatment groups. Although previous clinical trials showed improvements in psychological well-being and HRQL in patients treated with omeprazole compared with ranitidine,14-15 the study by Oster and colleagues18 showed few differences in psychological well-being. Omeprazole has been associated with improvements in psychological well-being and HRQL.15, 30-32 However, not all studies have shown differences in HRQL outcomes between patients with GERD who were treated with omeprazole or ranitidine. Our HRQL findings are not surprising, given the naturalistic study design, presence of multiple medical comorbidities, and the fact that only 44% (56 of 127 patients) of the omeprazole and 42% (50 of 119 patients) of the ranitidine group received treatment for 8 weeks or more.
The total medical costs associated with omeprazole treatment were $915 lower than those associated with ranitidine treatment, although this difference was not statistically significant. The omeprazole group had a $40 higher outpatient medical cost compared with the ranitidine group. These medical cost outcomes were similar to the findings in the study by Oster et al.18
During the study, patients covered by Medicaid were subject to an evolving prior authorization program that restricted access to PPIs and H2RAs in a general effort to reduce overall medical costs. This policy caused many study subjects to experience difficulty getting their prescriptions filled, particularly among the omeprazole group. Many of the patients covered by Medicaid initially randomized to omeprazole were subsequently shifted to other agents during weeks 8 through 12 under this policy.
Our study demonstrated that patients treated with omeprazole for more than 8 weeks reported more improvements in heartburn resolution after 3 or 6 months compared with ranitidine-treated patients or patients who discontinued omeprazole treatment before 8 weeks. Patients who started receiving omeprazole but discontinued the treatment before 8 weeks reported improvements in heartburn symptoms during the first month (41 [58%] of 71 patients) and heartburn resolution rates comparable to those of the ranitidine-treated groups at 3 and 6 months. Although the policy decision resulted in lower outpatient medical costs, these outpatient costs were not significantly different from those of the ranitidine-treated groups or the group treated with omeprazole for at least 8 weeks. In fact, total medical costs were comparable or lower in the group treated with omeprazole for at least 8 weeks compared with the other 3 groups.
There are some limitations associated with this clinical trial. First, patients and physicians were aware of study medications, which may have affected patient outcomes and physician management. Our intent, however, was to perform a completely naturalistic clinical trial, and unblinded treatment is necessary to generalize completely to usual-practice settings.20 Second, the introduction of nonprescription ranitidine while our study was ongoing may have biased clinical outcomes against ranitidine. However, nonprescription ranitidine is given at a lower dosage, and there is some evidence that continued treatment with ranitidine hydrochloride at higher dosages (ie, 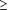 300 mg/d) is necessary to provide clinical efficacy against GERD.12-13 Third, during the course of our study, the West Virginia state government restricted omeprazole use in patients covered by Medicaid. This resulted in many patients discontinuing omeprazole treatment between the 4- and 12-week assessments and may have reduced the early-observed significant treatment differences in heartburn resolution and GERD-related symptoms. 300 mg/d) is necessary to provide clinical efficacy against GERD.12-13 Third, during the course of our study, the West Virginia state government restricted omeprazole use in patients covered by Medicaid. This resulted in many patients discontinuing omeprazole treatment between the 4- and 12-week assessments and may have reduced the early-observed significant treatment differences in heartburn resolution and GERD-related symptoms.
CONCLUSIONS
We found that omeprazole and ranitidine treatment resulted in significant short-term improvements in resolution of heartburn and reduction in GERD-related symptoms. Although previous studies have demonstrated a connection between GERD symptoms and HRQL,3, 15, 27 we did not observe any improvement in HRQL outcomes. Despite the difference in medication costs, the omeprazole outpatient group costs were only $40 greater, and total costs were $915 lower than those of the ranitidine group. Although not labeled by the Food and Drug Administration for long-term management of symptomatic GERD, our findings suggest that policy recommendations limiting omeprazole therapy for patients with GERD to 8 weeks or less may not result in appreciable savings in medical costs and may result in worse clinical outcomes in patients. This study provides evidence that omeprazole and ranitidine improve clinical symptoms in patients with GERD and that there are no significant differences in medical costs during 6 months in patients treated with ranitidine or omeprazole.
AUTHOR INFORMATION
Accepted for publication March 29, 2000.
Funding for this study was provided through an unrestricted research grant from AstraZeneca LP, Wayne, Pa.
Presented as a poster at the annual meeting of the American College of Gastroenterology, Phoenix, Ariz, October 15-20, 1999, and as a paper at the Second Annual Workshop on Pharmaceutical Outcomes Research at the annual meeting of the Drug Information Association, Seattle, Wash, May 12, 2000.
Corresponding author: Barbara Kaplan-Machlis, PharmD, Department of Clinical Pharmacy, West Virginia University, Robert C. Byrd Health Sciences Center, 3110 MacCorkle Ave SE, Charleston, WV 25304 (e-mail: bmachlis{at}hsc.wvu.edu).
From the Departments of Clinical Pharmacy (Dr Kaplan-Machlis) and Family Medicine (Dr Kaplan-Machlis and Mr Spiegler), West Virginia University, Charleston; and the Center for Health Outcomes Research, Medtap International, Bethesda, Md (Mr Zodet and Dr Revicki).
REFERENCES
 |  |
1. Nebel OT, Fornes MF, Castell DO. Symptomatic gastroesophageal reflux: incidence and precipitating factors. Am J Dig Dis. 1976;21:953-956.
FULL TEXT
|
ISI
| PUBMED
2. Locke GR, Talley NJ, Fett S, et al. The prevalence and impact of gastroesophageal reflux disease in the United States: a population-based study [abstract]. Gastroenterology. 1994;106:A15.
3. Revicki DA, Wood M, Maton P, Sorensen S. The impact of gastroesophageal reflux disease on health-related quality of life. Am J Med. 1998;104:252-258.
FULL TEXT
|
ISI
| PUBMED
4. Kaplan-Machlis B, Spiegler GE, Revicki DA. Health-related quality of life in primary care patients with gastroesophageal reflux disease. Ann Pharmacother. 1999;33:1032-1036.
ABSTRACT
5. Fennerty MB, Castell D, Fendrick AM, et al. The diagnosis and treatment of gastroesophageal reflux disease in a managed care environment. Arch Intern Med. 1996;156:477-482.
FREE FULL TEXT
6. Kahrilas PJ. Gastroesophageal reflux disease. JAMA. 1996;276:983-988.
FREE FULL TEXT
7. Sontag S, Robinson M, McCallum RW, et al. Ranitidine therapy for gastroesophageal reflux disease. Arch Intern Med. 1987;147:1485-1491.
FREE FULL TEXT
8. Sherbaniuk R, Wensel R, Bailey R, et al. Ranitidine in the treatment of symptomatic gastroesophageal reflux disease. J Clin Gastroenterol. 1984;6:9-15.
ISI
| PUBMED
9. Johansson K-E, Boeyrd B, Johansson K, Tibbling L. Double-blind crossover study of ranitidine and placebo in gastro-oesophageal reflux disease. Scand J Gastroenterol. 1986;21:769-778.
ISI
| PUBMED
10. Rush DR, Stelmach WJ, Young TL, et al. Clinical effectiveness and quality of life with ranitidine vs placebo in gastroesophageal reflux disease patients: a clinical experience network (CEN) study. J Fam Pract. 1995;41:126-136.
ISI
| PUBMED
11. Sandmark S, Carlsson R, Fausa O, Lundell L. Omeprazole or ranitidine in the treatment of reflux esophagitis: results of a double-blind, randomized, Scandinavian multicenter study. Scand J Gastroenterol. 1988;23:625-632.
ISI
| PUBMED
12. Maton PN. Drug therapy: omeprazole. N Engl J Med. 1991;324:965-975.
ISI
| PUBMED
13. Richter JE, Sabesin SM, Kogut DG, Kerr RM, Wruble LD, Collen MJ. Omeprazole vs ranitidine or ranitidine/metoclopramide in poorly responsive symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 1996;91:1766-1772.
ISI
| PUBMED
14. Revicki DA, Sorensen S, Maton PN, Orlando RC. Health-related quality of life outcomes of omeprazole versus ranitidine in poorly responsive symptomatic gastroesophageal reflux disease. Dig Dis. 1998;16:284-291.
FULL TEXT
|
ISI
| PUBMED
15. Wiklund I, Bardhan KD, Muller-Lissner S, et al. Quality of life during acute and intermittent treatment of gastro-oesophageal reflux disease with omeprazole compared with ranitidine: results from a multicentre clinical trial. Ital J Gastroenterol Hepatol. 1998;30:19-27.
ISI
| PUBMED
16. Bate CM, Green JRB, Axon ATR, et al. Omeprazole is more effective than cimetidine for the relief of all grades of gastro-oesophageal reflux disease–associated heartburn, irrespective of the presence or absence of endoscopic oesophagitis. Aliment Pharmacol Ther. 1997;11:755-763.
FULL TEXT
|
ISI
| PUBMED
17. Vigneri S, Termini R, Leandro G, et al. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106-1110.
FREE FULL TEXT
18. Oster G, Thompson D, Kaatz S, et al. The GERD outcomes trial: effectiveness and costs of omeprazole vs ranitidine in empiric treatment of heartburn in primary care practice [abstract]. Gastroenterology. April 1998;114(pt 2):A32.
19. Hillman AL, Bloom BS, Fendrick M, Schwartz JS. Cost and quality effects of alternative treatments for persistent gastroesophageal reflux disease. Arch Intern Med. 1992;152:1467-1472.
FREE FULL TEXT
20. Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world: effectiveness vs efficacy studies. Pharmacoeconomics. 1999;15:423-434.
FULL TEXT
|
ISI
| PUBMED
21. Williams DB, Welage LS. Gastroesophageal reflux. In: DePiro JT, Talbert RL, Hayes PE, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 3rd ed. Amsterdam, the Netherlands: Elsevier Science Publishers; 1997:675-696.
22. Svelund J, Sjodin L, Dotevall G. GSRS: a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci. 1988;33:129-134.
FULL TEXT
|
ISI
| PUBMED
23. Revicki DA, Wood M, Wiklund I, Crawley J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual Life Res. 1998;7:75-83.
FULL TEXT
|
ISI
| PUBMED
24. Dupuy HJ. The Psychological General Well-Being (PGWB) Index. In: Wender NK, Mattson ME, Furberg CD, Elinson J, eds. Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York, NY: Le Jacq; 1984:170-183.
25. Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston, Mass: Health Institute, New England Medical Center; 1993.
26. Revicki DA, Leidy NK, Howland L. Evaluating the psychometric characteristics of the Psychological General Well-Being Index with a new response scale. Qual Life Res. 1996;5:419-425.
FULL TEXT
| PUBMED
27. Revicki DA, Zodet MW, Crawley JA, Levine DS, Joelsson B. Complete resolution of heartburn symptoms and health-related quality of life in patients with gastroesophageal reflux disease. Aliment Pharmacol Ther. 1999;13:1621-1630.
FULL TEXT
|
ISI
| PUBMED
28. Ware JE Jr, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston, Mass: Health Institute, New England Medical Center; 1994.
29. Red Book 1998 Drug Topics. Montvale, NJ: Medical Economics Books; 1998.
30. Mattsson C, Lundell L, Nystrom P, Wiklund I. Quality of life (QoL) in the long-term management of gastroesophageal reflux disease treated with omeprazole (OME) or antireflux surgery (ARS): results of a multicentre randomized clinical trial [abstract]. Qual Life Res. 1998;7:635.
31. Carlsson R, Dent J, Watts R, et al. Gastro-oesophageal reflux disease in primary care: an international study of different treatment strategies with omeprazole. Eur J Gastroenterol Hepatol. 1998;10:119-124.
ISI
| PUBMED
32. Havelund T, Lind T, Wilkund I, et al. Quality of life in patients with heartburn but without esophagitis: effects of treatment with omeprazole. Am J Gastroenterol. 1999;94:1782-1789.
FULL TEXT
|
ISI
| PUBMED
|