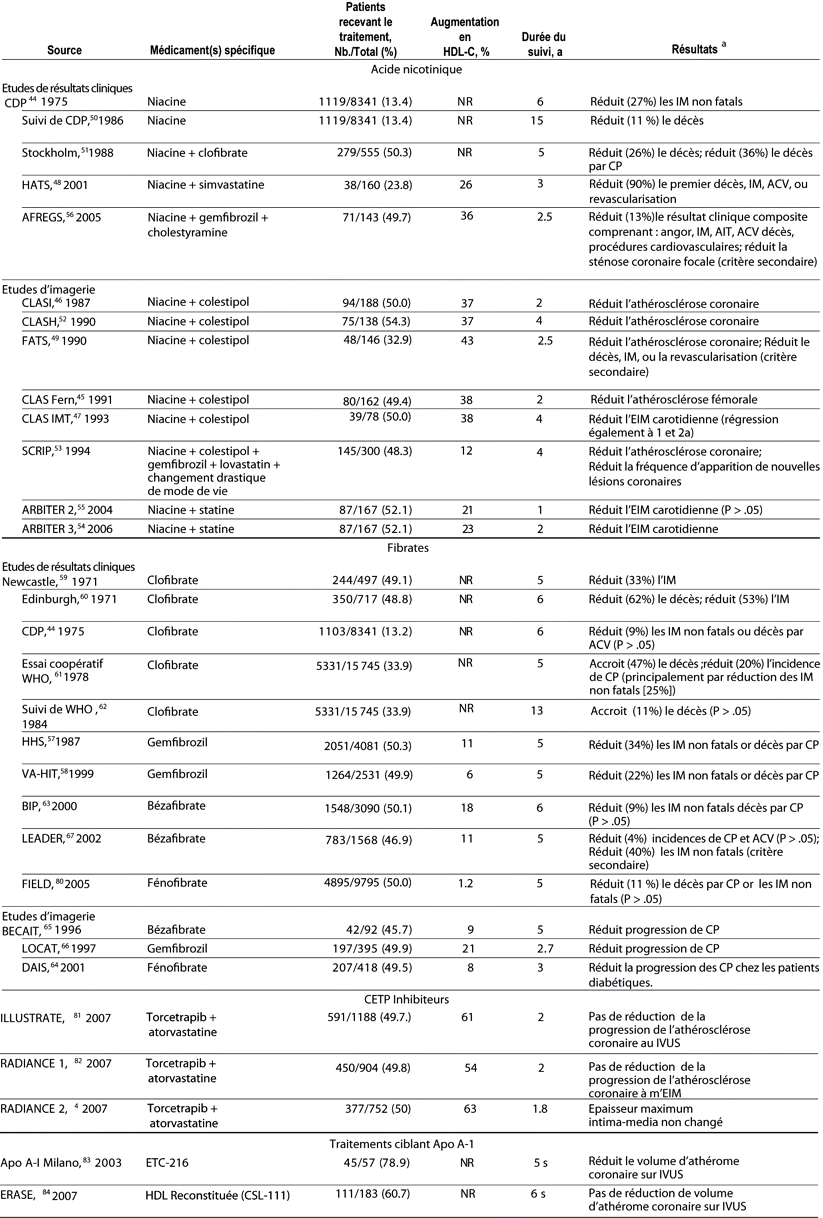
Table 3.. Essais randomisés contrôlés de traitements
médicamenteux modifiant HDL et impactant les résultats cliniques
ou le degré d'athérosclérose en critère de
substitution
Abréviations: AFREGS, Armed Forces Regression Study; apo A-1,
apolipoprotéine A-l; ARBITER, Arterial Biology for the Investigation of
the Treatment Effects of Reducing Cholesterol; BECAIT, Bezafibrate Coronary
Atherosclerosis Intervention Trial; BIP, Bezafibrate Infarction Prevention
Study; CP coronaropathie ; CDP, Coronary Drug Project; CLAS, Cholesterol
Lowering Atherosclerosis Study; CLAS Fern, groupe CLAS de
l'athérosclérose fémorale; CLAS IMT, groupe CLAS de la
carotide à l'ultrason; DAIS, Diabetes Atherosclerosis Intervention
Study; ERASE, Effect of rHDL on Atherosclerosis-Safety and Efficacy Trial;
FATS, Familial Atherosclerosis Treatment Study; FIELD, Fenofibrate
Intervention and Event Lowering in Diabetes Study; HATS, HDL-Atherosclerosis
Treatment Study; HDL-C, cholestérol associé aux
lipoprotéines de haute densité; HHS, Helsinki Heart Study;
ILLUSTRATE. Investigation of Lipid Level Management Using Coronary Ultrasound
to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation
Trial; EIM, épaisseur de l'intima-media; IVUS, échographie
intravasculaire; LEADER, Lower Extremity Arterial Disease Event Reduction
Trial; LOCAT, Lopid Coronary Angiography Trial; MI, infarctus du myocarde; NR
non rapporté; RADIANCE, Rating Atherosclerotic Disease Change by
Imaging with a New CETP Inhibitor Trial; SCRIP, Stanford Coronary Risk
Intervention Project; AIT, attaque ischémique transitoire; VA-HIT,
Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; WHO,
World Health Organization.; ACV attaque cérébrovasculaire
a Le décès correspond à la mortalité
globale.