
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Signaling Actions of Electrophiles: Anti-inflammatory Therapeutic Candidates
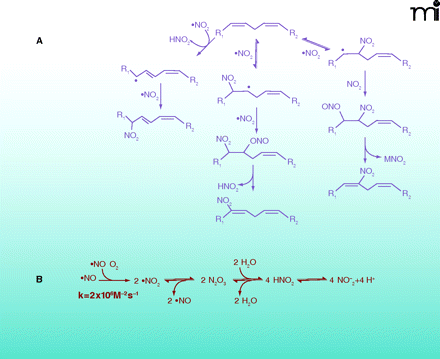
Multiple mechanisms can lead to the nitration of lipids. A. Biologically relevant NO2-FA products resulting from reaction of PUFA with NOX and reactive oxygen species. B. Reactive intermediates from autoxidation of •NO and acidification of nitrite. Under aerobic conditions •NO can react rapidly with O2 to form •NO2. When the O2 concentration is significantly lower than atmospheric conditions, •NO2 and •NO can be in equilibrium with N2O3. In aqueous milieu, N2O3 can be in equilibrium with HNO2. Finally, HNO2 is in equilibrium with is conjugate base NO2− (pKa=3.35). As all of these reactions are reversible, their order and equilibria depend on the source and site of production of NOX, the concentrations of various intermediates, and their reaction with other biomolecules.


