
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Mechanisms of Pathogenesis in Drug Hepatotoxicity Putting the Stress on Mitochondria
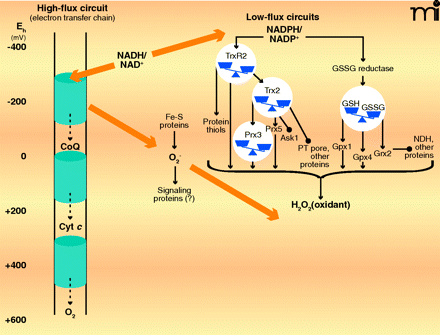
High-flux and low-flux systems of electron transfer pathways. High-flux systems (left), which support ATP production, transfer electrons into the inner membrane electron transfer chain via NADH and electron-carrying proteins such as Coenzyme Q (CoQ) and cytochrome c (Cyt c). The electron transfer to flavin occurs as an electron pair, but the electron transfer chain comprises single-electron transfers until the terminal reduction of molecular O2 to produce two molecules of H2O. At least ninety-nine percent of electron flow in the electron transfer chain results in the reduction of molecular O2. A small fraction of the total flux, probably ≤1% under most physiologic conditions, results in the single-electron reduction of molecular O2 to yield superoxide anion radical (O2•−). The superoxide anion is rapidly dismutated to H2O2 and O2, and the potential role of superoxide as a signaling molecule, rather than merely as unavoidable byproduct of aerobic respiration, remains under investigation. Certain low-flux pathways, which function in signaling and cell regulation, are well established (right), although many more are likely to be elucidated. One of the major NADPH-dependent pathways involves thioredoxin reductase-2 (TrxR2), thioredoxin-2 (Trx2), and the peroxiredoxins-3 and -5. This pathway depends upon H2O2 as an oxidant and regulates apoptosis by means of signal-regulating kinase-1 (Ask1) and the permeability transition (PT) pore. Another major NADPH-dependent pathway involves the mitochondrial isoform of GSSG reductase, GSH, GSH peroxidase-1 and -4, and glutaredoxin-2 (Grx2). Grx2 controls S-glutathionylation of NADH dehydrogenase (NDH) and other proteins. H2O2 and lipid peroxides also function in oxidation of the GSH pathway (not shown). Some iron-sulfur proteins (e.g., aconitase) are also directly oxidized by O2•− The low-flux pathways have been extensively studied as mechanisms to protect against oxidative stress. The fraction of total electron flow diverted to NADPH to support detoxification has not been firmly established. [Approximate scale for steady-state redox potentials (Eh) is indicated at the far left; the thiol/disulfide systems operate between the potential of the NADPH/NADP+ couple (−400 mV) and that of the H2O2/H2O couple (approx +100 mV).]


