Bisphosphonate Therapeutics in Bone Disease: The Hard and Soft Data on Osteoclast Inhibition
Abstract
Bisphosphonates are chemically stable structural analogs of inorganic pyrophosphate. Owing to their ability to inhibit osteoclast activity, bisphosphonates have become the primary agents to treat conditions marked by excessive osteoclast-mediated bone resorption, such as osteoporosis, Paget’s disease of bone, and cancer-associated bone disease. At the molecular level, bisphosphonates exert their anti-resorptive effects by inhibiting farnesyl pyrophosphate synthase activity within osteoclasts. Bisphosphonates are generally well tolerated, with many patients now having been treated for more than ten years. Widespread bisphosphonate use, however, has revealed both short-term and long-term side effects in some patients. Here, we review our current understanding of the mechanisms by which bisphosphonates inhibit osteoclast function, the current roles for these medications in clinical practice, and areas of concern that have emerged with widespread bisphosphonate use.
Introduction
Bisphosphonates were first synthesized over a century ago for industrial use as corrosion inhibitors, reflecting their ability to inhibit the growth of calcium deposits (1). It was not until the late 1960s, however, that bisphosphonates became appreciated as important modulators of bone and calcium metabolism (2). Bisphosphonate drugs are now used across the human lifespan to limit osteoclast-mediated skeletal resorption and treat a variety of conditions, including heritable skeletal disorders in children, age-related and corticosteroid-induced osteoporosis in both men and women, hypercalcemia of malignancy, and metastatic bone disease. The recognition of bisphosphonate use may be associated with low bone–turnover states that result in fractures, osteonecrosis of the jaw, and renal impairment has brought renewed scrutiny to this widely used drug class. In this review, we describe the development of bisphosphonates as therapeutic agents, the proposed mechanisms by which bisphosphonates exert their effects, and the current roles for bisphosphonates in clinical practice. We also review the growing list of potential adverse effects associated with bisphosphonate use and highlight some issues associated with bisphosphonate use which are currently unresolved.
Chemical Structure of Bisphosphonates
Pioneering studies from the 1960s demonstrated that inorganic pyrophosphate, generated as a by-product of many enzymatic reactions, could inhibit calcification by binding to hydroxyapatite crystals; this observation suggested that skeletal mineralization might be regulated via modulation of pyrophosphate levels (3). As structural analogs of pyrophosphate, bisphosphonates became the focus of investigation to determine their ability to affect hydroxyapatite crystal formation. In a series of seminal observations published just over forty years ago, Fleisch and colleagues demonstrated that like, inorganic pyrophosphate, bisphosphonates could indeed inhibit hydroxyapatite dissolution. Moreover, bisphosphonates were found, both in vitro and in vivo, to inhibit hydroxyapatite breakdown and thereby suppress bone resorption (4).
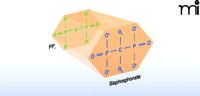
Although bisphosphonates are structural analogs of pyrophosphate (Figure 1), they lack the phosphodiester linkage that makes pyrophosphate so labile to hydrolysis. All bisphosphonate drugs contain a central carbon atom bound directly to two phosphorous atoms, which explains their chemical stability relative to pyrophosphate. Both phosphonate moieties are essential for bisphosphonate binding to hydroxyapatite crystals in bone and for osteoclast inhibition (5, 6). Bisphosphonates are further distinguished by the two side chains (denoted R1 and R2) bound to the central carbon. For the vast majority of bisphosphonates in current clinical use (including the potent nitrogen-containing bisphosphonates), R1 is a hydroxyl group (Table 1). Collectively, the phosphonate and hydroxyl groups establish a tertiary rather than a binary interaction between drug and hydroxyapatite, which is reflected in bisphosphonate affinity for bone matrix skeletal specificity. The bisphosphonate R2 moiety is the primary determinant of potency in osteoclast inhibition (Figure 1). As shown in Table 1, a nitrogen- containing R2 side chain can increase anti-resorptive potency by up to 10,000-fold relative to bisphosphonates lacking a nitrogenous R2 side chain (7, 8). Recent in vitro findings, along with computational modeling studies, corroborate a role for the R2 group in determining bisphosphonate affinity for hydroxyapatite (6).
The general molecular structure of bisphosphonates. A planar representation of bisphosphonate stresses the structural analogy between bisphosphonate drug and pyrophosphate. (Side chains R1 and R2 that occur in bisphosphonate drugs are detailed in Table 1.)
Bisphosphonate Structures and Relative Activities
Mechanism of Action
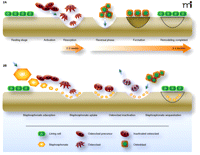
Normal osteoclast-mediated bone resorption is closely coupled, both spatially and temporally, with osteoblast-mediated bone formation (Figure 2A). High avidity for bone relative to other tissues is a critical feature shared by all bisphosphonates and allows them to achieve high local concentrations throughout the skeleton following adsorption. As seen in Figure 2B, bisphosphonates preferentially distribute to sites in the skeleton with increased bone turnover, and this preferential distribution may reflect the greater availability of hydroxyapatite binding sites relative to quiescent bone. Once bound to bone, bisphosphonates may be released by dissolution or osteoclast-mediated bone resorption, or they may become embedded within the bone itself as osteoblasts function to lay down new tissue.
The dynamics of bone remodeling and mechanisms of bisphosphonate intervention. A) In the normal process of bone remodeling, the breakdown of bone matrix (resorption) is tightly coupled, both spatially and temporally, with regeneration of matrix (formation) in a highly dynamic process. The activation of osteoclast precursor cells results in the ruffled-border multinucleate morphology (i.e., the active osteoclast) that penetrates the bone lining and comes into contact with bone. Resorption is schematized here as a scalloped erosion (wells). Osteoblasts deposit bone matrix, which undergoes subsequent mineralization, and thereby function in concert with osteoclasts to maintain a healthy, dynamic skeleton. B) Bisphosphonates (shown as orange hexagons) intervene in the resorption process (as indicated by the relatively shallow wells) and can function to counteract disease processes such as osteoporosis. As pyrophosphate analogs, bisphosphonates associate tightly with hydroxyapatite exposed during osteoclast-mediated bone resorption. Bisphosphonates are taken up by osteoclasts and function as inhibitors of FPP synthase and promote osteoclast apoptosis. Bisphosphonates that bind to hydroxyapatite can also become embedded in the bone matrix by osteoblast function and bone mineralization (indicated here as “sequestration”). Embedded bisphosphonates can then be released by the normal bone cycle activities, depending on the prevalent rates of bone resorption and remodeling, and function in subsequent rounds of osteoclast inactivation. (Purple arrows indicate movement of cells or molecules relative to the bone surface.)
Bisphosphonates that remain embedded in bone mineral for prolonged periods are eventually released into acidic lacunae generated during osteoclast-mediated bone resorption. Once liberated into the resorption lacunae, bisphosphonates are taken up by osteoclasts, likely via simple fluid-phase endocytosis (9). Fluorescently labeled bisphosphonates that are pharmacologically active have allowed Rogers and colleagues to monitor bisphosphonate concentrations within local microenvironments and detect bisphosphonate flux amid bone surface and osteoclasts (10).
Extreme tissue selectivity and potent osteoclast inhibition account for the current use of bisphosphonates as agents of choice in the treatment of skeletal disorders marked by excessive osteoclast activity. More recently, it has become apparent that bisphosphonates may also play a role in reducing apoptosis of osteocytes (i.e., terminally differentiated osteoblasts) that are entombed in the very bone matrix they had laid down for mineralization. Intriguing studies suggest that bisphosphonates may protect osteocytes from glucocorticoid- or fatigue-induced apoptosis (11, 12) likely via inducing conduction through connexin 43 hemichannels within osteocytes and activating extracellular signal regulated kinase (ERK) signaling (13). The relative importance of earlier reports suggesting that bisphosphonates may also limit osteoblast apoptosis (11) is less clear.
As shown in Table 1, the early bisphosphonates (etidronate, clodronate, and tiludronate) are characterized by R2 side chains that lack nitrogen-containing groups. Because they closely resemble pyrophosphate, these bisphosphonates are recognized as substrates by type II amino-acyl-transfer-RNA synthetases, and subsequent to uptake from the bone mineral surface by osteoclasts, they are thereby converted into analogs of ATP (14). It has thus been hypothesized that the intracellular accumulation of non-hydrolyzable ATP analogs, synthesized from the non-nitrogenous bisphosphonates, is the basis for bisphosphonate cytotoxicity and the induction of osteoclast apoptosis.
In contrast, alendronate, pamidronate, ibandronate, risedronate, and zoledronate bear R2 side chains that contain nitrogen atoms (Table 1). Relative to the early bisphosphonates discussed above, this chemical distinction, which dramatically increases bisphosphonate potency for osteoclast inhibition, fundamentally alters the mechanism by which this newer class of bisphosphonates acts. Rather than serving, like their predecessors, as substrates for the generation of non-hydrolyzable ATP analogs, the nitrogen-containing bisphosphonates appear to function primarily through inhibition of farnesyl pyrophosphate synthase (FPPS), a key regulatory enzyme in the mevalonic acid pathway for the synthesis of cholesterol, other sterols, and isoprenoid lipids (8, 15, 16). In addition, there is limited data to suggest that suppression of FPPS activity may not be the sole mechanism through which nitrogen-containing bisphosphonates inhibit bone resorption (17).
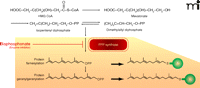
The inhibitory binding of nitrogen-containing bisphosphonates to the peroxisomal enzyme FPPS has been studied in some detail (15, 18). As shown in Figure 3, FPPS inhibition has important downstream metabolic effects, including a decrease in the generation of both farnesyl diphosphate and geranylgeranyl diphosphate, post-translational moieties that direct the subcellular membrane localization of multiple proteins, including small G proteins (e.g., Rab, Rac, and Rho) that regulate stress fiber assembly, membrane ruffling, and osteoclast survival (19). Thus, whereas FPPS inhibition per se may initially lead to impairment of osteoclast function, bisphosphonate exposure over time results in osteoclast apoptosis, although the apoptotic process appears to be protracted in some cases (20).
Biochemical mechanism of nitrogen-containing bisphosphonates in osteoclast inactivation. The mevalonate pathway supports sterol and isoprenoid lipid synthesis as well as the posttranslational isoprenylation of proteins (indicated as green spheres). Nitrogen-containing bisphosphonates bind to FPPS, thereby inhibiting enzymatic activity (red blunt arrow). FPPS inhibition thus prevents the subsequent posttranslational modification (isoprenylation) of multiple small GTP-binding proteins (Rab, Rac, and Rho) central to osteoclast function, ultimately leading to osteoclast apoptosis.
Importantly, although FPPS is ubiquitously expressed in mammalian cells and plays a crucial role in lipid production, general cellular apoptosis does not occur on a large scale in response to nitrogen-containing bisphosphonates, which reflects the extreme selectivity of bisphosphonates for mineralized tissue. Bisphosphonates embedded in bone remain accessible for osteoclast endocytosis and are thus able to achieve high local concentrations uniquely within the osteoclast cytoplasm. As the vast majority of bisphosphonate therapy currently involves the more potent, nitrogen-containing-bisphosphonates, we will focus on this class of bisphosphonates for the remainder of this review.
Additional Properties of Bisphosphonates
Skeletal uptake and retention of systemic bisphosphonate are dependent upon host factors pertaining to renal function and bone turnover, in addition to the properties of bisphosphonate binding to bone matrix (21). Accordingly, the amount of bisphosphonate retained following oral or intravenous administration varies significantly across patients, clinical conditions, and bisphosphonate species (21). Responses to therapy, such as decreased bone loss, reduced fracture risk, and increased bone mineral density, occur over prolonged periods (months to years), and are thus not generally apparent immediately after starting treatment. The most direct method for assessing the skeletal effects of bisphosphonates is through bone biopsy; however, this highly invasive approach provides information about only a small fraction of the skeleton. For less invasive assessment of bisphosphonate efficacy and potency, serum and urine biochemical markers of bone resorption (e.g., N- and C-terminal breakdown products of type 1 collagen) have been used as surrogate markers (22). Measurement of bone resorption markers demonstrates that maximal osteoclast suppression occurs by roughly three months, whether oral bisphosphonate therapy is started daily, weekly, or monthly, and remains roughly constant with continuation of treatment (21, 23, 24). Suppression of bone resorption occurs more rapidly after intravenous bisphosphonate administration.
The duration of bisphosphonate-mediated bone resorption is likely a function of the drug’s affinity for mineral matrix as well as its potency in inhibiting FPPS. Accordingly, intravenous administration of zoledronate [four to five milligrams (25, 26)], a bisphosphonate with relatively strong mineral binding properties and highest efficacy as an FPPS inhibitor, leads to suppression of biochemical bone resorption markers in postmenopausal women for at least one year. Intravenous delivery to such women of an equimolar dose of a pamidronate, a less potent FPPS inhibitor, leads to less and shorter-lasting suppression of bone resorption.
The half-lives of nitrogen-containing bisphosphonates remain the subject of some debate, reflecting the complex and reiterative interplay between bisphosphonate and bone. Whereas roughly 50% of absorbed bisphosphonate is excreted unchanged in the urine within a few hours of administration in subjects with normal renal function, the strong affinity of bisphosphonates for hydroxyapatite in bone mineral causes the remaining (unexcreted) drug to clear rapidly from the circulation to the bone surface, where it largely becomes embedded during the mineralization process (27). The embedded drug remains essentially inactive until it is recycled to the bone surface by osteoclast-mediated resorption of bone mineral. Current estimates for alendronate, a widely used bisphosphonate drug, suggest a biological half-life of greater than ten years after delivery of a single intravenous dose (28). It is tempting to combine characteristics such as skeletal half-life, anti-resorptive potency, and recycling kinetics of bone-embedded species to explain both the long-term effects of bisphosphonates on bone resorption and variations among specific bisphosphonates (21). At the moment, however, such attempts to explain long-term observations remain largely conceptual.
More recently, the time variability at which different bisphosphonates effectively limit fracture risk has been described. As articulated by Russell and colleagues, such variability may reflect differences in microanatomical distribution among different bisphosphonates, such that bisphosphonates with comparatively lower bone mineral binding affinity have a comparatively broader area of skeletal distribution and thus perhaps a more rapid effect on limiting fracture risk (6). The relative contribution of the microanatomical distribution of bisphosphonates to their effects on bone turnover, however, remains to be clarified.
Although not widely recognized, a critical feature governing the clinical pharmacology of bisphosphonates is their extremely low bioavailability. As a class, bisphosphonates are markedly hydrophilic, with paracellular transport required for essentially all absorption after oral dosing. Accordingly, bioavailability following oral administration is extremely limited, estimated to be approximately 1% (21, 29). Moreover, this limited bioavailability is also highly prone to interactions with ingested solids and liquids, especially those containing even modest amounts of calcium (e.g., coffee with milk). These interactions can reduce bisphosphonate bioavailability to zero.
Bisphosphonates can be taken daily, weekly, monthly, or less frequently, depending on the particular species and mode of delivery. The introduction of intravenous bisphosphonate preparations (e.g., pamidronate, ibandronate and zoledronate) for a variety of common clinical conditions has helped to overcome impediments to compliance, namely, oral administration requiring patients to remain upright for thirty minutes and refrain from eating before (two hours) and after (at least thirty minutes) pill ingestion. Gastrointestinal side effects have also been relatively common among some patients managed with oral bisphosphonates. On the other hand, intravenous administration of nitrogen-containing bisphosphonates manifests an increased rate, relative to oral administration, of acute phase reactions marked by transient flu-like symptoms (e.g., low-grade fevers, myalgias/arthralgias, and headaches) (26). Whether intravenous bisphosphonate preparations will become the delivery method of choice in the future is not clear, although there are distinct advantages for certain patient populations.
Bisphosphonates in Clinical Practice
Many pathologic skeletal conditions are characterized by excessive osteoclast-mediated bone resorption. These include multiple types of osteoporosis, including juvenile, age-associated (both male and female), glucocorticoid-induced, transplant-induced, immobility-induced, androgen-deprivation-related types. Other such conditions are Paget’s disease of bone, osteogenesis imperfecta, osteoclast-induced hypercalcemia, and metastatic bone disease. All nitrogen-containing bisphosphonates suppress osteoclast activity (as assessed by biochemical markers of bone resorption), although with differing efficacies. As described above, such variability likely relates to differences in bisphosphonate ability to inhibit FPPS as well as differences in bisphosphonate affinity for bone matrix. Superior suppression of bone resorption markers, however, does not always appear to correlate with effects in fracture risk (6). In fact, some data suggest that increased adherence to long-term bisphosphonate therapy, independent of the specific bisphosphonate utilized, may be important in determining effectiveness on reduction of fracture risk (30, 31).
Age-Associated Osteoporosis
Bisphosphonates are the most frequently used medications for the treatment of osteoporosis, a skeletal condition characterized by compromised bone strength and increased fracture risk. Although osteoporosis is often considered a condition that affects elderly women, it is in fact a clinically heterogeneous disease with a wide array of etiologies, many of which may pertain to the individual patient. Postmenopausal osteoporosis results from declining estrogen levels, which lead to increases in osteoclast-mediated bone resorption, deterioration of bone microstructure, and increased fracture risk. By effectively suppressing osteoclast activity, bisphosphonates have become the primary agents for the treatment and prevention of postmenopausal osteoporosis over the past two decades.
In postmenopausal women with osteoporosis, the orally available bisphosphonates alendronate and risedronate have been shown to decrease the incidence of vertebral (32–34), hip (32, 35), and other fractures. Similar results have also been shown for the intravenous bisphosphonate zoledronic acid (26). Studies of ibandronate (available in oral or intravenous preparations) have demonstrated a reduction in vertebral fracture risk (36, 37), and post hoc analyses have also shown a reduction in non-vertebral, but not hip fractures. Finally, although most studies of age-related osteoporosis have focused on women, trials of men with low bone mass have in general demonstrated similar responses to bisphosphonate therapy (38–40).
Glucocorticoid-Induced and Transplant-Associated Osteoporosis
Although corticosteroids are among the most commonly prescribed medications, the association of bone loss with the prolonged use of these immunosuppressive agents remains under-recognized, with most patients failing to receive any therapy to combat bone loss (41). Bisphosphonates are highly effective at limiting skeletal losses both in patients treated with corticosteroids and in subjects undergoing transplantation. Alendronate, risedronate, ibandronate, pamidronate, and zoledronate have all been shown effective at limiting bone loss (42). Further, in glucocorticoid-treated patients at high risk for fracture, including those with a prior history of fracture, rheumatoid arthritis, or those receiving high glucocorticoid doses, bisphosphonate therapy is cost-effective (43). In addition, numerous studies have shown that both oral and intravenous bisphosphonates can limit bone loss associated with either solid organ (44–48) or bone marrow transplantation (49–51).
Paget’s Disease of Bone
Paget’s disease of bone is marked by focal areas of accelerated osteoclast-mediated bone resorption that is poorly matched by osteoblast-mediated bone deposition (52). Poorly formed woven bone is generated, frequently associated with pain, increased fracture risk, and marked skeletal deformity, including bowing of weight-bearing long bones and skull enlargement. Since their introduction to clinical practice, bisphosphonates have been used successfully to limit the osteoclast-mediated bone resorption (frequently with normalization of the serum alkaline phosphatase levels used to assess disease activity). Both oral (alendronate and risedronate) and intravenous (pamidronate and zoledronate) bisphosphonates have received FDA approval for the treatment of Paget’s disease of bone (53).
Cancer-Associated Bone Disease
The skeleton is the most common site of metastatic disease, with upwards of 90% of patients with advanced cancer having skeletal lesions (54). Cancers may metastasize to bone or grow within the bone marrow, leading to hypercalcemia, severe bone pain, skeletal destruction, and pathologic fractures. These complications from metastatic bone disease are sometimes referred to as skeletal-related events. Another form of cancer-associated bone disease is bone loss that results from cancer treatment itself.
Breast Cancer
For patients with breast cancer metastatic to bone, intravenous pamidronate (55, 56), zoledronate (57, 58), or ibandronate (59) can be used to limit pain and reduce skeletal complications. Further, bisphosphonate treatment has been shown to limit bone loss in breast cancer patients who had been depleted of estrogen as part of their cancer therapy (60, 61). Finally, recent studies of women with breast cancer but no evidence of skeletal metastases suggest that bisphosphonates may have an adjunctive role in limiting disease progression (62, 63).
Prostate Cancer
Although skeletal lesions in prostate cancer have classically been considered to result from increased osteoblast activity, the importance of increased osteoclast-mediated bone resorption has more recently been appreciated (64). In men with hormone-refractory prostate cancer, zoledronate treatment reduces skeletal-related events by 11% at two years relative to placebo (65, 66). In addition, men with hormone-responsive prostate cancer who undergo androgen deprivation therapy are at increased risk for bone loss, which can be limited by alendronate, risedronate, pamidronate, or zoledronate therapy (67).
Multiple Myeloma
Multiple myeloma is characterized by the clonal proliferation of malignant plasma cells within the bone marrow cavity, leading to osteoclast-mediated skeletal destruction with resultant fractures. Both pamidronate and zoledronic acid reduce the hypercalcemia as well as the incidence of skeletal-related events associated with myeloma (68–70) and are central adjunctive agents to current treatment regimens.
Other Malignancies
Although many malignancies metastasize to bone, the routine use of prophylactic bisphosphonate therapy has been little studied. Interestingly, however, bisphosphonate therapy was recently shown to delay the onset and progression of skeletal disease in patients with renal cell carcinoma (71), suggesting that patients with malignancies that less frequently metastasize to bone may also benefit from bisphosphonate therapy.
Bisphosphonate Treatment of Children
Although rarely used in children, bisphosphonates have recently become the primary pharmacological agents to treat osteogenesis imperfecta, a skeletal disorder marked by severely diminished bone mass and extreme fragility, usually resulting from heritable mutations in the genes for type I collagen. Both oral alendronate and, more commonly, cyclic intravenous pamidronate have been used. Both have been shown to impart substantial increases in bone mineral density and decreases in fracture incidence (72).
Although other illnesses in children, including cystic fibrosis, rheumatoid arthritis, and anorexia nervosa, can have significant deleterious effects on the skeleton, data on the routine use of bisphosphonates in these populations are extremely limited. Accordingly, a recent systematic review of this topic concluded that current evidence does not support bisphosphonate use as standard therapy, although treatment for three years or less appears to be well-tolerated (73).
Adverse Effects of Bisphosphonate Therapy
Following FDA approval of alendronate in 1995, bisphosphonates became widely utilized in clinical practice, primarily for the treatment of age-related bone loss in women, but also for the many other indications described above. Although some side effects, such as gastroesophageal irritation and nephrotoxicity were recognized early as potential adverse effects in clinical trials, other more serious potential adverse effects associated with bisphosphonate use have only recently become widely appreciated.
Osteomalacia
The ability of bisphosphonates both to inhibit mineralization and reduce bone resorption established, particularly for early compounds such as etidronate, a relatively narrow therapeutic window. The dosages that inhibited skeletal mineralization (leading to osteomalacia) differed by as little as one or two orders of magnitude from dosages that inhibited bone resorption. The subsequent development of bisphosphonates with nitrogen-containing R2 substituents has considerably widened the therapeutic window, so that inhibition of skeletal mineralization is no longer of significant concern clinically with regard to these newer drugs.
Upper Gastrointestinal Side Effects
Upper gastrointestinal side effects are the most common reason patients are intolerant of oral bisphosphonate therapy, and strict guidelines for oral bisphosphonate administration have been developed. These include ingestion with a full glass of water and maintenance of an upright posture for thirty to sixty minutes thereafter. Accordingly, multiple studies show that the incidence of nausea, dyspepsia, abdominal pain, and gastritis in oral bisphosphonate administration is similar to placebo (74).
Esophageal Cancer
A recent statement from the FDA describes that oral bisphosphonate use may also be associated with an increased risk of esophageal cancer (75). Specifically, the FDA received reports of twenty-three patients taking alendronate who were diagnosed with esophageal cancer between 1995, when the drug was approved, through mid 2008. Median time to diagnosis was 2.1 years from bisphosphonate initiation. Further reports of 31 patients from Europe and Japan demonstrated that in addition to alendronate, esophageal cancer occurred in patients prescribed risedronate, ibandronate, and etidronate, with a median time to diagnosis of 1.3 years. Further study will be needed to determine whether the development of cancer in these reports represents non-adherence to prescribing directions with associated esophageal irritation or erosion. Notably, several of the affected patients had a known pre-malignant esophageal condition (Barrett’s esophagus) prior to starting therapy, suggesting extreme caution should be used when considering oral bisphosphonates in this population.
Musculoskeletal Pain
Although musculoskeletal pain is noted as a potential adverse effect in the prescribing information for all bisphosphonate drugs, a recently issued FDA alert has highlighted the possibility of severe and sometimes incapacitating bone, joint, and/or musculoskeletal pain that can occur any time after bisphosphonate initiation (76). Although discontinuation improves symptoms in some affected patients, symptom resolution appears to be slow or incomplete in some cases. The risk factors and incidence of these potential adverse bisphosphonate-associated effects are at present unknown.
Hypocalcemia
Transient hypocalcemia is an under-appreciated consequence that occurs most frequently with intravenous bisphosphonate preparations. Susceptible subjects appear to be those with unrecognized hypoparathyroidism or hypovitaminosis D, impaired renal function, limited calcium intake, or high rates of osteoclast-mediated bone resorption (such as subjects with Paget’s disease of bone or a large skeletal tumor burden). In a study that measured serum calcium in patients with cancer complicated by bone metastases, total serum calcium levels declined an average of 2 mg/dL by seven days and nearly 3 mg/dL by twenty-one days after zoledronate infusion (77), with concomitant compensatory rises in serum parathyroid hormone levels. Accordingly, assessment to ensure adequate calcium and vitamin D intake should be performed prior to starting bisphosphonate therapy.
Acute Phase Reactions
Intravenous (and rarely oral) bisphosphonate therapy may be associated with a transient (24–72 hour) acute phase reaction characterized by fever, myalgias, arthralgias, and malaise. In clinical trials with zoledronate, 1 in 3 subjects had such a reaction with the first infusion, although incidence declined progressively with subsequent infusions (1 in 15 subjects with a second infusion; 1 in 35 subjects with a third infusion) (26). This adverse effect, which appears to be less common with intravenous ibandronate, is thought to reflect the release of TNF-α by γδ-T cells that are possibly activated by isopentyl pyrophosphate, the metabolite immediate upstream of FPPS in the mevalonate pathway (78). Of note, this reaction is idiosyncratic, and symptoms may be mitigated by pretreatment with acetaminophen.
Ocular Inflammation
Ocular inflammation (including uveitis, conjunctivitis, episcleritis and scleritis, ocular pain, and photophobia) has been reported as a rare complication of both oral and intravenous bisphosphonate preparations. Onset is idiosyncratic, occurring weeks, months, or even years following bisphosphonate initiation, and necessitates ophthalmologic referral. Resolution of ocular symptoms usually occurs within a few weeks after bisphosphonate discontinuation (74).
Osteonecrosis of the Jaw
Recent recognition that osteonecrosis of the jaw (ONJ) may occur with bisphosphonate therapy has focused scrutiny on the entire drug class. Although based on incomplete data, current estimates of ONJ related to oral bisphosphonate therapy for osteoporosis are approximately 1-per-10,000 to 1-per-100,000 patient treatment years (79). Importantly, the incidence of ONJ in patients with malignancy is much higher, likely reflecting the significantly higher cumulative bisphosphonate doses administered to oncology patients. In such patients, ONJ incidence has been estimated to be 1 to 10 per 100 patients, although the higher rates have generally been reported prior to the standardization of ONJ case definition (79). Higher rates occur in subjects with poor oral hygiene, those who require invasive dental procedures, and those with higher cumulative bisphosphonate exposure (80). More recent studies suggest that prophylactic efforts (e.g., treatment of tooth decay prior to beginning therapy) can substantially reduce the incidence of ONJ in oncology patients. Further, a recent report suggests that antibiotic prophylaxis for invasive dental procedures occurring while receiving bisphosphonate therapy may reduce ONJ incidence, at least in patients with multiple myeloma (81). Care for ONJ is largely supportive, with antiseptic oral rinses, antibiotics, and limited surgical debridement as necessary. Potential evidence that the risk for ONJ is related to serum levels of bone resorption markers such as CTX is insufficient.
Atrial Fibrillation
The recent recognition that bisphosphonate therapy may be associated with an increased risk of atrial fibrillation was first noted in the HORIZON study of postmenopausal women who received once-yearly intravenous zoledronate or placebo (26). In that study, women who received zoledronate had a statistically significant increase (relative risk 1.3% vs 0.5%) in the incidence of “serious” atrial fibrillation (defined as events leading to hospitalization or disability or judged to be life-threatening), although rates of serious vs non-serious events did not differ between groups. Although subsequent analyses of the Fracture Intervention Trial (82) suggested that alendronate use may also be associated with a slightly increased risk of atrial fibrillation, this finding was not confirmed in other large studies of alendronate (83) or risedronate (84). More recently, however, a meta-analysis, including all of these studies, showed that serious atrial fibrillation occurred more frequently in bisphosphonate-treated subjects, with a relative risk of 1.525 (85). Importantly, a unifying mechanism to account for this risk has not been defined, nor has an effect of bisphosphonate dose or duration been demonstrated.
Excessive Suppression of Bone Turnover
After start of an oral bisphosphonate, a decrease of bone resorption markers is followed temporally by a decline in bone formation markers. Whereas the former usually reaches a nadir at three months, the latter reaches a nadir at approximately six months after treatment initiation (21, 22). Thereafter, levels of both bone resorption and formation markers usually remain consistently suppressed until discontinuation of therapy.
Given the profound ability of bisphosphonates to inhibit osteoclast activity, a potential concern of prolonged bisphosphonate therapy is that excessive suppression of bone remodeling could impair the normal reparation of skeletal micro-fractures and result in skeletal fragility. Although micro-fractures have been found in canine models of high-dose bisphosphonate therapy, oversuppression of bone remodeling does not appear at present to be a common problem in postmenopausal women treated with either oral or intravenous bisphosphonate therapy for osteoporosis (79). However, rare cases have been reported of atypical fractures occurring with bisphosphonate therapy, biopsies in several instances demonstrating severely suppressed bone turnover (86, 87).
On the other hand, two studies failed to show an increase of fracture risk or bone remodeling abnormalities after ten years of alendronate therapy (88, 89). Importantly, however, these studies included less than 1000 total subjects (including fewer than 5% who underwent bone biopsy). Thus, it remains plausible, albeit rare, that some patients may have significant oversuppression of bone turnover with prolonged bisphosphonate therapy. Because bone formation is normally closely coupled with bone resorption, it is at present unclear whether measurement of markers of bone resorption or formation will provide the most useful information in subjects in whom oversuppression of bone turnover is suspected.
Subtrochanteric Femoral Fractures
As with the other less common adverse effects associated with bisphosphonate therapy, subtrochanteric femoral fractures were not identified in clinical trials, but have recently come to clinical attention based on an expanding collection of case reports of prolonged bisphosphonate therapy (90–92). These fractures typically occur in the proximal or mid-femoral diaphysis with minimal or no trauma and are transverse or oblique (< 30º). They are often preceded by thigh pain, vague discomfort, or subjective localized weakness prior to fractures, which heal slowly (93, 94). Interestingly, imaging of the contralateral femur shaft may show thickened cortices and the presence of a cortical stress reaction.
At present, no algorithms are available to identify patients at increased risk for this type of fracture after initiation of bisphos-phonate therapy. Although a recent FDA alert highlights the current uncertainty surrounding these fractures and their relationship to bisphosphonate use (95), a secondary analysis of three large randomized bisphosphonate trials concluded that there was no significant increase in the risk of subtrochanteric or diaphyseal femoral fractures with bisphosphonate use, but noted the study was underpowered for definitive conclusions (96).
Kidney Dysfunction
Bisphosphonate doses and infusion rates require adjustment in patients with diminished renal function. Although rare, intravenous bisphosphonate infusion has been associated with acute deterioration of renal function (97, 98), an outcome that may reflect local bisphosphonate accumulation within the kidney. Interestingly, different pathologic mechanisms for the observed nephrotoxicity have been described for different bisphosphonates, including collapsing glomerulonephritis for pamidronate and acute tubular necrosis for zoledronate (99). For patients with compromised renal function, kidney function both before and subsequent to intravenous bisphosphonate administration should be followed; slow infusion of dilute bisphosphonate preparations reduce the risk for nephrotoxicity. Oral bisphosphonates rarely lead to further deterioration of renal function in patients with mild to moderate renal impairment, likely reflecting their poor intestinal absorption and consequently limited acute bioavailability.
Conclusion
The widespread introduction of bisphosphonates has revolutionized clinical approaches to a wide array of skeletal disorders characterized by excessive osteoclast-mediated bone resorption and has significantly decreased patient morbidity. Here, we have reviewed the mechanisms by which bisphosphonates inhibit osteoclast activity and discussed some areas of concern for clinical practice. We have described some recent concerns about bisphosphonates and have reviewed a wealth of data from well-designed clinical trials that demonstrate that for the vast majority of patients, the appropriate use of bisphosphonates confers a clear clinical benefit. The remarkable translation of in vitro and animal studies from just forty years ago is reflected in the millions of patients who currently benefit from bisphosphonate therapy.
- Copyright © 2010
References

Matthew T. Drake, MD, PhD, is Assistant Professor of Medicine at the College of Medicine, Mayo Clinic in Rochester, Minnesota. His current work is focused on understanding the basis by which monoclonal gammopathies induce human bone disease. In his clinical endocrinology practice, he is involved in providing care to patients with a wide variety of metabolic bone diseases. Send correspondence to MTD. E-mail drake.matthew{at}mayo.edu; fax (507) 284-9111.

Serge C.L.M. Cremers, PharmD, PhD, is Assistant Professor of Medical Sciences at the College of Physicians and Surgeons of Columbia University Medical Center in New York, New York. He is Director of the Division of Endocrinology’s Bone Marker Laboratory and the Biomarker Core Laboratory of the Irving Institute for Clinical and Translational Research, home of Columbia’s Clinical and Translational Science Award. His work focuses on clinical chemistry, toxicology, and biochemical markers of bone turnover.