The Secrets of a Successful Clinical Trial: Compliance, Compliance, and Compliance
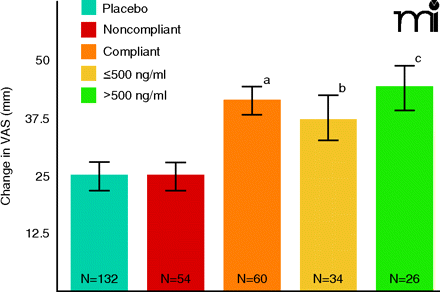
These data represent patients who received either a controlled release form of bicifadine (200 mg, twice daily) or placebo. The visual analog scale (VAS) ranges from 0 100 mm. For patient recruitment in the study reported here, baseline pain severity had to be at least 40 mm. Changes in VAS score was the primary endpoint measure; analysis of plasma bicifadine in this trial arm was a planned pharmacokinetic/pharmacodynamic comparison. VAS change scores as endpoint measures were compared with ANCOVA. Severity at baseline, gender and age were applied as covariates. The pattern of results shown here was largely replicated (data not shown) using the Roland-Morris Disability Questionnaire (15); see text for details. (ap<0.01 vs placebo or noncompliant groups; bp<0.05 vs placebo or noncompliant groups; cp<0.002 vs placebo or noncompliant groups).



