
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Determining the Destiny of NF-κ B after TCR Ligation: It’s CARMA1
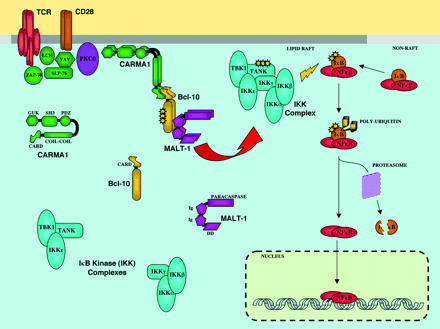
Figure 1. A Model of TCR/CD28-Induced NF-κB Regulation.
The coligation of the TCR with CD28 results in the activation of tyrosine kinases and the recruitment of lipid rafts to the aggregated TCR. Several receptor proximal tyrosine kinases, adaptors, and effectors, including Lck, ZAP-70, SLP-76, and Vav, have been implicated in the regulation of NF-κB and the recruitment of PKC?? to lipid rafts. CARMA1, specifically required for the activation of NF-κB by the TCR and PKC??<links raft-associated receptor complexes to Bcl-10 through the heterodimerization of the CARMA1 and Bcl-10 CARD domains. CARMA1 membrane association is most likely driven by the MAGUK homology region, which consists of a PDZ domain, a SH3 domain, and a guanylate kinase (GUK) homology domain. The recruitment of Bcl-10 results in its phosphorylation, with unknown consequences. Bcl-10 directs the multimerization of the human paracaspase MALT1, which interacts with Bcl-10 through its immunoglobulin-like (Ig) domains. Multimerization of the MALT1 paracaspase domain is sufficient to activate NF-κB by unknown mechanisms. Ultimately, NF-κB activation is triggered by the induced phosphorylation, ubiquitinylation, and proteasomal degradation of IκB. The canonical IκB kinase (IKK) complex—consisting of the kinases IKKα and IKKβ, and the linker IKKγ—is required for the initiation of this degradative process. Preliminary evidence suggests that additional IκB kinases (TBK1, IKKε) and scaffolds (TANK) participate in the degradation of IκB in response to TCR ligation or phorbol ester and calcium ionophore treatment. TCR, T cell receptor; ZAP-70, TCRζ-associated protein of 70-kD; IKK, inhibitor-of-κB kinase; SLP-76, SH2 domain-containing leukocyte protein of 76-kD; CARMA1, caspase recruitment domain-containing MAGUK protein-1; MALT1; mucosa-associated lymphoid tissue of B lymphoma 1; nuclear factor-κB, NF-κB; TANK, tumor necrosis factor receptor-associated factor (TRAF) family member-associated NF-κB activator; TBK1, TANK-binding kinase 1.


