
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
The “Heartbreak” of Older Age
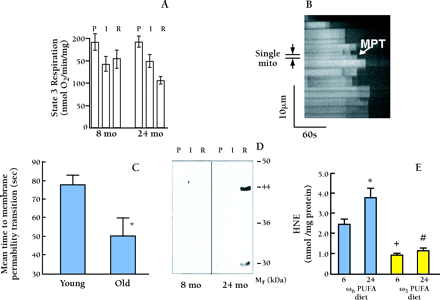
Experimental assessment of membrane-permeablity transistion in individual in situ mitochondria by photoexcited ROS production. A. Age-associated differences in the effect of ischemia and reperfusion on ADP-dependent mitochodrial respiration. Mitochondria were isolated and respiratory activities were assessed; glutamate supported State 3 respiration after perfusion (P), ischemia (I), or reperfusion (R) of hearts from 8- and 24-month-old rats. From (45) . B . Confocal linescan imaging of fluorescence in tetramethylrhodamine methyl ester (TMRM) loaded single rat cardiac myocyte, at 2 Hz along a 25 μ m longitudinal segment encompassing approximately twenty mitochondria; time progresses from left to right. TMRM fluorescence serves as the index of mitochondrial membrane potential, ΔΨ . The apparent dark regions between horizontal columns are extra mitochondrial spaces between individual mitochondria (approximately half-sarcomere spacing). The sudden dissipation of TMRM fluorescence (i.e., white-to-black transitions at certain points in time) in individual mitochondria indicates the loss of ΔΨ due to MPT induction. A representative individual mitochondrion is delimited showing its MPT occurrence after approximately 90 sec of photoexcitation. C. Mitochondria from cardiac myocytes of aged (24-month old) rats are more susceptible to ROS-induction of the MPT vs. those from young (3-month old). Cells were examined as in Panel A , and represent the average from 8–10 cells in each group. D. SDS/gel electrophoresis and Western blot analysis of protein from cardiac mitochondria in the experiment in Figure 8A. Only mitochondria from 24-month-old reperfused hearts exhibited protein modified by hydroxynoneal (HNE). Thus, HNE-induced change of mitochondrial enzymes might underlie the exaggerated age-associated decline in mitochondrial state 3 respiration during reperfusion observed in Panel A . From (45) . HNE was detected by HPLC assay in cardiac mitochondria isolated from 6- and 24-month-old rats treated to either ω3 PUFA or ω6 PUFA rich diets for 6 weeks. Hearts (n=8) were subjected to 15-min low-flow global ischemia and reperfused for 30 min prior to isolation of mitochondria. HNE was detected in mitochondria isolated from normoxic controls at a concentration less than 1 pmol/mg mitochondrial protein. E. HNE was detected by HPLC assay in cardiac mitochondria isolated from 6- and 24-month-old rats treated to either ω3 PUFA or ω6 PUFA-rich diets for 6 weeks. Hearts (n=8) were subjected to fifteen minutes of low-flow global ishcemia and reperfused for thirty minutes prior to isolation of mitochondria. HNE was detected in mitochondria isolated from normoxic controls at a concentration less than 1 pmol/mg mitochondrial protein.


