
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Rapid Extranuclear Signaling by the Estrogen Receptor (ER): MNAR Couples ER and Src to the MAP Kinase Signaling Pathway
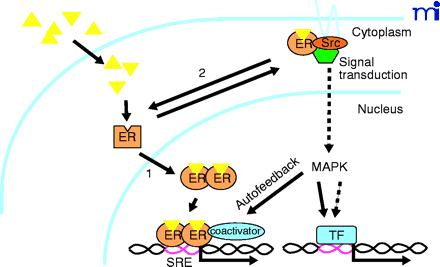
Model of classical and “nongenomic” actions of the estrogen receptor (ER). Estrogenic ligands (triangles) activate ER as a transcription factor (1) by inducing a conformational change(s) that leads to nuclear translocation, dimerization, and binding to specific steroid receptor response elements (SRE) in promoters of primary target genes. Activated ER recruits coactivators that are essential for assembly of a productive transcription complex at the promoter. Alternatively, a subpopulation of ER associates in an estrogen-dependent manner (2) with cytoplasmic- and/or cell membrane–signaling molecules including the tyrosine kinase Src. This extranuclear interaction promotes the Shc-Src-Raf–MAPK kinase (MEK)–MAP kinase phosphorylation cascade. Because MAPK can directly (solid arrows) or indirectly (dashed arrows) activate other transcription factors (TF), the MAP kinase pathway can potentially regulate distinct or complementary sets of genes from those regulated by nuclear ER pathways. Estrogen-activated MAPK can also enhance the direct transcriptional activity of ER by an autofeedback loop through phosphorylation of ER or ER-associated coactivators. The hexagon indicates an adaptor protein.


