
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Portrait of a Killer: The Mitochondrial Apoptosome Emerges From the Shadows
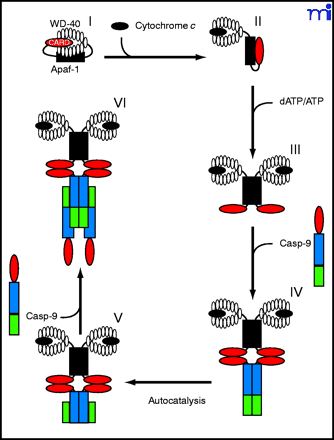
Hypothetical model illustrating the role that cytochrome c and dATP binding play in regulating apoptosome assembly. In the absence of cytosolic cytochrome c, Apaf-1 may exist in a compact, autoinhibited form, with the Apaf-1 CARD region buried between the lobes of the WD-40 repeats (I). The binding of cytosolic cytochrome c then displaces the Apaf-1 CARD from the WD-40 repeats, forcing the molecule into a more open conformation (II). Binding of dATP to the Walker boxes within the CED-4 arm region creates a less flexible, locked conformation (III). This prevents reassociation between the hub and WD-40 repeats, facilitating apoptosome assembly. Caspase-9 (Casp-9) is recruited to the apoptosome via CARD-CARD interaction (IV). Binding to Apaf-1 results in an increase in caspase-9 activity, autoprocessing at D315, and separation of the large and small subunits (V). A free caspase-9 molecule is recruited to each of the Apaf-1–bound caspase-9 molecules; however, only one caspase-9 molecule within each caspase-9 heterotetramer may be catalytically active (VI).


