
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Targeting the FLICE Inhibitory Protein (FLIP) in Cancer Therapy
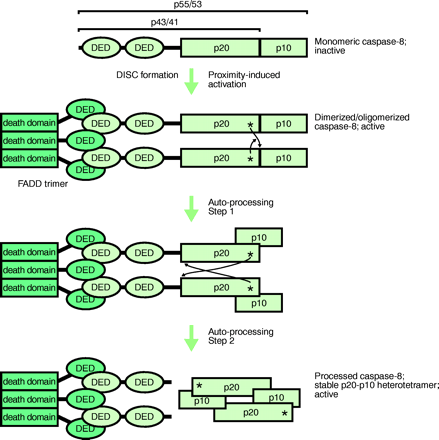
DISC-mediated activation of caspase-8.
Formation of the death-inducing signalling complex (DISC) initially activates unprocessed procaspase-8 by driving its dimerization. Subsequently, dimerized active procaspase-8 is autoproteolytically processed, attaining the mature heterotetrameric state of caspase-8 with DISC-independent activity. The thin arrows indicate proteolytic processing in trans, whereby the asterisks represent the active-site cysteine residues and the arrowheads point to the sites of proteolytic cleavage. However, upon their release by proteolysis—between the DEDs and the p20 subunits—the heterodimers orient themselves into a head-to-tail manner. FADD trimer bound to Fas (not shown). DED, death effector domain; FADD, Fas-associated death domain protein; p20 and p10 represent the subdomains of the caspase homology domain of caspase-8.


