Transgenic Analysis of Human Drug–Metabolizing Enzymes: Preclinical Drug Development and Toxicology
Abstract
The cytochromes P450 (CYPs) are drug-metabolizing enzymes (DMEs), and constitute a large family of monooxygenases, arising from multiple genes, capable of catalyzing an extraordinary range of biochemical reactions, from the synthesis of cholesterol, bile acids, and steroid hormones to the metabolism of drugs, prodrugs, and xenobiotics. The CYPs, along with other DMEs, remain the subject of intense scientific research not only to ascertain their functions in vivo, but also to better understand how the expression of these enzymes is regulated. Transgenic technologies are providing important animal models for studying how DMEs—including the particularities of human DMEs—provide an adaptive response to cellular stress, establishing relationships between polymorphisms and disease susceptibility, and investigating how CYPs and other DMEs determine the pharmacological action of therapeutic drugs1.
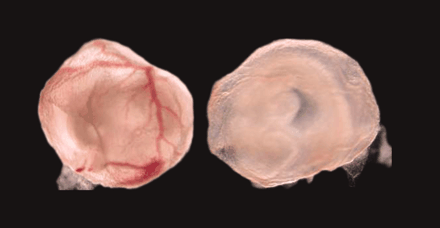
The lack of multiple cytochrome P450 activities in early development can be studied in a transgenic mouse. Most striking here
is the effect on blood vessel formation in the yolk sac of transgenic mice that lack cytochrome P450 reductase (right), relative
to wild type (left) 23).
- © American Society for Pharmacology and Experimental Theraputics 2003



