
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
sorLA: Sorting Out APP
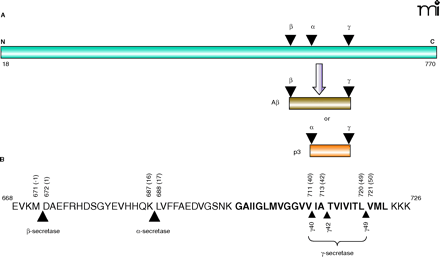
Proteolysis of amyloid precursor protein. A. The membrane-spanning protein amyloid precursor protein (APP) (red) first undergoes ectodomain shedding (i.e., proteolytic cleavage) at either the site of β- or α-secretase activity. This ectodomain shedding process generates the immediate substrate for γ-secretase, which cleaves within the transmembrane domain of APP. Aβ (dark gold) is a product of β- and γ-secretase cleavages. Cleavage by α-secretase (before β-secretase has a chance to cleave APP) results in the peptide termed p3 (dark orange) and precludes the formation of Aβ. The first seventeen amino acids constitute the signal peptide and are not shown. B. Amino-acid residues of Aβ and surrounding region of human APP770 showing the β-, α-, and γ- proteolytic sites. γ-Secretase cleaves at multiple sites within the transmembrane region. Protein sequence is numbered from the first Met. Aβ sequence is indicated in bracket. The transmembrane domain is shown in bold.


