Emerging concepts from the recent literature
Thinking thin
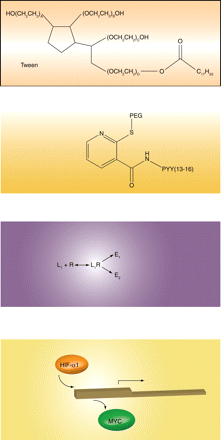
The growing obesity epidemic brings urgency to the search for new therapeutic options. Limited clinical success in targeting energy metabolism in muscle, liver, and adipocytes has recently widened investigator focus to include neurological pathways that regulate body weight. The neuropeptide Y (NPY) family of G protein–coupled receptors has been implicated in the regulation of satiety (NPY2 and NPY4) and feeding (NPY1 and NPY5). Peptide YY (3–36), an anorexigenic gut hormone and nonselective NPY receptor agonist, was recently used to design a family of shorter peptides with several N-terminal modifications. One of these peptides, with an N-terminal polyethyleneglycol (PEG) moiety, is characterized in the November issue of JPET. The PEGylated peptide causes dose-dependent weight loss in lean rodents, an effect that is blocked by a selective NPY2 antagonist. The PEG moiety is absolutely required for long-term stability and responsiveness. Once-daily administration of the peptide for extended periods increases plasma adiponectin and improves glucose disposal and plasma insulin levels, all of which are important factors in type II diabetes. These results validate ongoing efforts to target the neuronal basis of appetite and weight management. [Ortiz, A.A., Milardo, L.F., DeCarr, L.B., Buckholz, T.M., Mays, M.R., Claus, T.H., Livingston, J.N., Mahle, C.D., and Lumb, K.J. A novel long-acting selective neuropeptide Y2 receptor PEGylated peptide agonist reduces food intake and body weight and improves glucose metabolism in rodents.J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.107.125211 (2007).]—C Beeson, Medical University of South Carolina
Life and death in the hippocampus
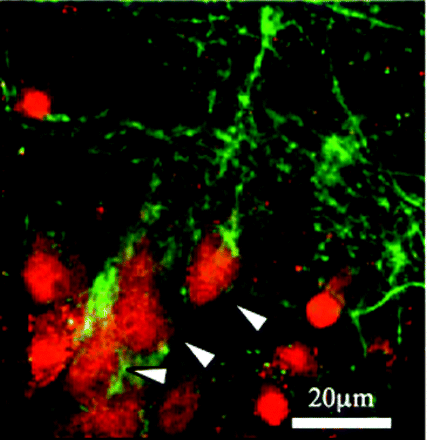
Cerebral ischemia is a leading cause of morbidity and death. Loss of blood fl ow during ischemic events results in rapid depletion of oxygen and glucose, which deregulates ion currents and thereby undermines basic neuronal function. Cavaliere et al. have previously shown that microglial and neuronal purinergic (P2) receptors are involved in pathways that signal deprivation of oxygen and glucose. In the November issue of JPET, the authors now delineate that P2X1, a member of the ionotropic P2X receptor subfamily, is expressed on hippocampal neurons, and its expression, moreover, is induced by ischemic conditions. Levels of P2X1 appear to be associated with cell death, and indeed, the P2 receptor antagonist TNP-ATP, which reduces neuronal cell death, accordingly reduces P2X1 expression. These results suggest that specifi c modulators of P2X1 receptors might have a role in the treatment of ischemic damage to the brain. [Cavaliere, F., Amadio, S., Dinkel, K., Reymann K.R., and Volonté, C. P2 receptor antagonist trinitrophenyl-ATP protects hippocampus from oxygen and glucose deprivation cell death. J. Pharmacol. Exp. Ther. Epub ahead of print; doi: 10.1124/jpet.107.125211 (2007).] — C Beeson, Medical University of South Carolina
Meddling with MYC mucks up multiplication
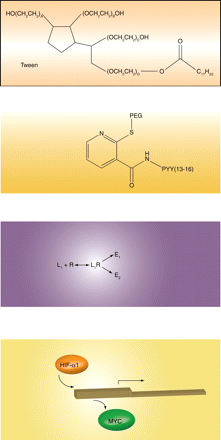
A major effector of gene expression in response to decreased oxygen tension is hypoxia inducible factor 1α (HIF-1α), a transcription factor rapidly degraded under normoxic conditions but stabilized under low oxygen tension. Upon stabilization, HIF-1α translocates to the nucleus, regulating oxygen-sensitive gene expression. Previously, investigators had shown that HIF-1α displaces MYC from the promoter of the CDKN1A gene, thereby derepressing expression of the CDK2 inhibitor p21Cip1 and leading to cell cycle arrest. Now, the same laboratory demonstrates an alternatively nuanced HIF-1α–mediated mechanism of cell cycle control. In this instance, HIF-1α appears to displace an activator form of MYC from the CDC25A promoter, thus downregulating expression of the cell cycle regulator CDC25A, a tyrosine phosphatase that otherwise maintains CDK2 activity. These fi ndings are consistent in suggesting that hypoxia, via HIF-1α, mediates cell cycle arrest by counteracting MYC-mediated regulation of transcription. Ultimately, these fi ndings may explain the ability of cells to continue to grow under hypoxic conditions and implicate Myc overexpression as a driver of aberrant proliferation in hypoxic cells. [
.] —PE Queiroz de Oliveira, University of Pittsburgh
Ligand-directed signaling at β-adrenoceptors
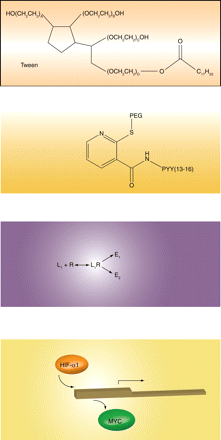
The term “ligand-directed signaling,” also known as “functional selectivity,” “biased agonism,” or “protean agonism,” describes the phenomenon that two drugs acting on the same receptor can elicit qualitatively different cellular responses. This phenomenon potentially paves the way for a ligand to specifi cally target one (desired) effect relative to another (adverse) effect of a given receptor. Sato et al. use β3-adrenergic receptors to report on two new twists of this theme. They show that ligands may selectively activate distinct proximal intracellular pathways, but both pathways can converge to yield the same distal response, indicating that ligand-directed signaling may occur more frequently than we thought. The authors also show that differences in receptor expression density may affect ligand-directed signaling. Although differences in physicochemical behavior (e.g., tissue partitioning) or other pharmacokinetic factors may explain many clinically observed differences between drugs that act on the same receptor, ligand-directed signaling is another potential explanation and has potential clinical relevance. [Sato, M., Horinouchi, T., Hutchinson, D.S., Evans, B.A., and Summers, R.J. (2007) Ligand-directed signaling at the β3-adrenoceptor produced by SR59230A relative to receptor agonists. Mol. Pharmacol. Epub ahead of print; doi:10.1124/mol.107.035337 (2007).] —MC Michel, University of Amsterdam
- © American Society for Pharmacology and Experimental Theraputics 2007



