
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
S-Glutathionylation: Indicator of Cell Stress and Regulator of the Unfolded Protein Response
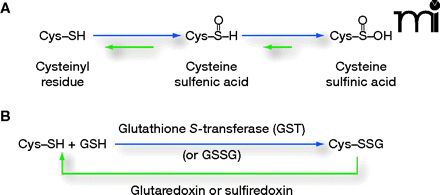
Reactivity of cysteinyl residues and the process of S-glutathionylation. A. The cysteinyl residues of proteins (Cys; Cys–SH to stress the thiol form) of cells under oxidative/nitrosative stress can be oxidized to various acidic forms, including cysteine sulfenic acid (Cys–SOH), which is fairly labile and can be readily reduced back to the thiol (Cys–SH) or further oxidized to the more stable cysteine sulfinic acid form (Cys–SOOH). It is becoming increasingly clear that such oxidation may be essential to normal deactivation-and-reactivation cycles of proteins and enzymes. B. The more familiar reaction of protein cysteinyl residues, namely, in the formation of disulfide bonds (e.g., as a manifestation of protein secondary structure), is related to the process of protein S-glutathionylation, which is increasingly recognized as essential to cellular behavior in health and in disease states. In the presence of physiological concentrations of glutathione (GSH), specific cysteinyl residues, by virtue of their position and reactivity within protein microenvironments, can undergo such modification through reactions with oxidized glutathione disulfide (GSSG) or by glutathione-utilizing enzymes such as glutathione S-transferases (GST). This posttranslational modification becomes reversible under catalysis that involves small redox proteins such as sulfiredoxin and glutaredoxin. (Oxidation is indicated in blue; reduction is indicated in green.)


