
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
An Old Dog Learns a New Trick: Regulation of Peripheral Glucose Homeostasis by the Serotonin (5-HT)2C Receptor
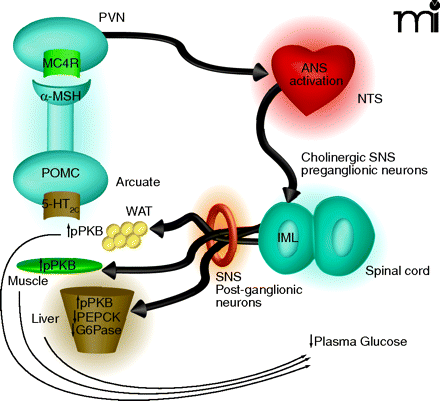
Proposed mechanism by which 5-HT2C receptor agonists regulate peripheral glucose. Activation of 5-HT2C receptors (5-HT2CRs) expressed on proopiomelanocortin (POMC) neurons of the arcuate would lead to elevated α-MSH release in the periventricular nucleus (PVN). Activation of MC4 receptors (MC4Rs) in the PVN would be followed by downstream activation of the autonomic nervous system (ANS), likely in the region of the nucleus of the solitary tract (NTS). Based on the findings by Zhou et al. (9) of c-Fos expression and co-localization with choline acetyltransferase (ChAT), the intermediolateral nucleus (IML) is likely a relay point between the CNS and periphery. As the IML is part of the sympathetic nervous system (SNS) one would expect that post-ganglionic neurons of the SNS are then activated to effect changes in the muscle, white adipose tissue (WAT) and liver that influence glucose production, sensitivity, or both. The increased level of protein kinase B phosphorylation (pPKB) observed in the three tissues is suggestive of improved insulin sensitivity. The 5-HT2C-mediated reductions in phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6P) mRNA in the liver are classically associated with enhanced insulin signaling in that organ and would be expected to result in reduced gluconeogenesis.


