Emerging concepts from the recent literature
Signal Duration at Parathyroid Hormone Depends on Conformation of Receptor
By using variants of the parathyroid hormone (PTH) peptide, the authors previously identified at least two regions on the parathyroid hormone receptor (PTHR) responsible for binding ligand. Results from that article and from new findings here led the authors to hypothesize that PTHR is capable of adopting either a GTPγS-resistant (R0 conformation) or a GTPγS-sensitive (RG conformation). PTH variants bound to PTHR R0 resulted in long-lasting complexes that produced increased cAMP-dependent signaling. Additionally, blood Ca2+ concentrations increased with a concomitant decrease in circulating phosphate. In vivo assessment of one particular modified ligand [M-PTH(1–34)] demonstrated that its administration increased bone volume and bone turnover more to a higher degree than did administration of unmodified PTH(1–34). Thus, targeting PTHR in vivo, through appropriate drug design of R0-favoring ligands that prolong signals favoring bone formation, may have useful clinical benefit in osteporosis [Okazaki, M., Ferrandon, S., Vilardaga, J.P., Bouxsein, M.L., Potts, J.T., Jr., and Gardella, T.J. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. U.S.A. Epub ahead of print; doi: 10.1073/pnas.0808750105 (2008).]
—JW Nelson, ASPET
Pharmacological Decoy Provides Insight into Proteasome Mechanics
The 26S proteasome, a 2.5 megadalton intracellular protease complex, is responsible for degrading the vast majority of cellular proteins and is indispensable for numerous cellular functions. The holoenzyme is composed of a barrel-shaped 20S core particle, which contains the proteolytic active sites, and the 19S regulatory particle, which caps the open ends of the 20S particle and recognizes, unfolds, and inserts the substrate proteins into the 20S for degradation. Because association of 19S with the 20S appears to be very weak, there has been some controversy over whether the 19S and 20S particles dissociate. Indeed, must they dissociate for protein degradation to occur? Kriegenburg et al. hypothesized that if 19S separated from 20S during each proteolytic cycle, they should be able to sequester freed 19S particle upon its release. The authors used epoxomicin to create inactive 20S “sink” particles and passed an excess amount of them over immobilized, active holoenzymes in the presence of a proteasome substrate. The rate of substrate proteolysis by the active enzymes was unchanged by the presence of the 20S “sink” particles, indicating that 19S and 20S remain intact during catalysis. [Kriegenburg, F., Seeger, M., Saeki, Y., Tanaka, K., Lauridsen, A.M., Hartmann-Petersen, R., and Hendil, K.B. Mammalian 26S proteasomes remain intact during protein degradation. Cell135, 355–365 (2008)].
—Tomko, Yale U
More for the CNS: Improving Intranasal Administration of Drugs
A recent report indicated that administration of hypocretin-1/orexin-A (HC) could significantly improve task performance by sleep-deprived monkeys. Although intravenous (iv) administration was beneficial, intranasal administration of orexin-A yielded a greater salutary effect on performance. One facet of intranasal application that could be improved is that of direct drug delivery into the CNS (via the olfactory and trigeminal networks) instead of into capillaries in the nasal mucus membrane. Dhuria et al. have identified a simple method for decreasing the amount of nasally delivered drug into the competing capillaries. The authors used 1% phenylephrine (PHE) in the intrasal application of radiolabeled HC or D-kyotorphin (D-KTP) and tested peripheral and CNS tissues and blood from rats thirty minutes after administration and clearance. Blood concentrations of HC and KTP were markedly lower when 1% PHE was used in the nasal spray. Additionally, the concentrations of HC and KTP were increased in the olfactory bulbs but decreased in the trigeminal nerves. Future studies should determine fully the kinetics of such peptide administration. Nonetheless, if applicable to patients, this methodology might be useful to reduce the adverse peripheral effects of potent neuropeptides. [Dhuria, S.V., Hanson, L.R., and Frey, W.H., II. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J. Pharmacol. Exp. Therap. Epub ahead of print; doi: 10.1124/jpet.108.145565 (2008).]
—W Nelson, ASPET
TRIs: Antidepressant Drugs v 2.0
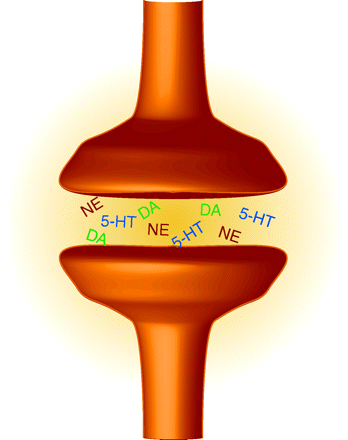
Selective serotonin reuptake inhibitors (SSRIs) are a powerful group of drugs that target depression; however, these might be replaced in the not so distant future by triple reuptake inhibitors (TRIs). These TRI antidepressants block not only the reuptake of serotonin, but that of norepinephrine and dopamine as well. Additionally, they might produce a more rapid onset of beneficial effect as well as exhibit increased efficacy and patient tolerability. Liang et al. evaluate a new TRI, PRC200-SS, finding that it binds to the human serotonin, norepinephrine, and dopamine transporters and inhibits their reuptake in cells expressing the transporters. In forced-swim tests, rats administered PRC200-SS exhibited decreased immobility (a model antidepressant activity). Increased extracellular concentrations of serotonin and norepinephrine in the medial prefrontal cortex, and of serotonin and dopamine in the core of nucleus accumbens, were noted when PRC200-SS was administered peripherally. When given access to PRC200-SS, rats did not appear to abuse it, suggesting a low abuse potential for humans. [Liang, Y., Shaw, A.M., Boules, M. et al. Antidepressant-like pharmacological profile of a novel triple re-uptake inhibitor, PRC200-SS. J. Pharmacol. Exp. Therap. Epub ahead of print; doi: 10.1124/jpet.108.143610 (2008).]
—JW Nelson, ASPET
- © American Society for Pharmacology and Experimental Theraputics 2008



