
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Targeted Delivery of Radioprotective Agents to Mitochondria
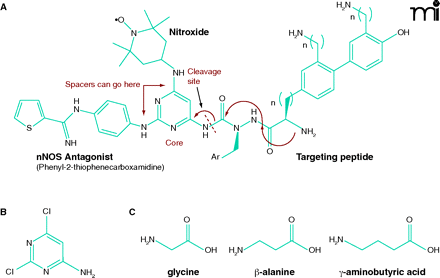
Figure 3.
Scheme for dual-action drug ligation using a pyrimidine core and amino acid spacer groups. A. As a core structure to ligate mtNOS inhibitors, ROS scavengers (e.g., TEMPO derivatives) and targeting peptide, one can choose a commercially available compound such as 4-amino-2,6-dichloropyrimidine B. The halogen atoms on this heterocycle can be selectively displaced with amine nucleophiles, and the amino function can serve as the attachment point for the targeting peptide. C. Additional bifunctional amino acid spacer groups (e.g., glycine, β-alanine or γ-aminobutyric acid) can be readily introduced (unpublished data).


