
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Targeted Delivery of Radioprotective Agents to Mitochondria
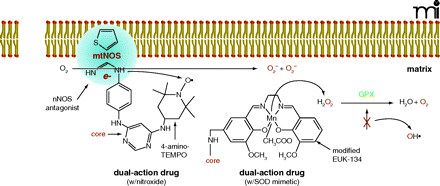
The right combination. Scheme for the mechanism of a dual-action drug with an NOS antagonist bound to NOS, resulting in electrons being transferred to O2 instead of the normal substrate, L-arginine. A pyrimidine core connects the NOS antagonist (e.g., Phenyl-2-thiopenecaraboxaminide) to a nitroxide (e.g., 4-amino-TEMPO) which can scavenge electrons and prevent O2− formation. An SOD chemically modified mimetic (e.g., EUK-134) may be used in place of the nitroxide derivative in the dual-action drug. However, because SOD mimetics scavenge and convert O2− to H2O2, the highly reactive OH radical may be formed if glutathione peroxidase (GPX) activity decreases. Therefore, a SOD mimetic that exhibits intrinsic GPX/catalase-like activity should be employed. Adapted from (18).


