Pharmacokinetic Assessment of Multiple ATP-binding Cassette Transporters: The Power of Combination Knockout Mice
Abstract
A TP-binding cassette (ABC) multidrug transporters are cellular efflux pumps with broad and often widely overlapping substrate specificities. They can have a major impact on the pharmacokinetics and hence overall pharmacological behavior of many drugs. To study their separate roles and functional overlap, or complementarity, a collection of mice deficient in two or more ABC transporters has been generated. This review discusses recent findings obtained with these models, focusing on pharmacokinetic studies with a number of clinically relevant drugs. In addition, the characterization of these mice and some physiological aspects of ABC multidrug transporters are addressed.
Introduction
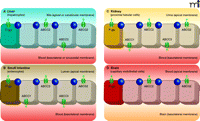
ATP-binding cassette (ABC) transporters, including the multidrug efflux transporters P-glycoprotein (P-gp; also known as ABCB1/ MDR1), ABCC1–3 (MRP1–3), and ABCG2 (or BCRP), are trans-membrane proteins that can actively extrude a wide variety of endogenous and exogenous compounds from cells. Most multi-drug ABC transporters have broad and substantially overlapping substrate specificities, and their substrates include many drugs and drug metabolites [reviewed in (1, 2)]. P-gp, ABCC2, and ABCG2 are localized at the canalicular membranes of hepatocytes and at the apical membrane of epithelial cells of the intestinal tract and kidney; their distribution at these membrane locations allow these transporters to pump their substrates into bile, feces, and urine. Consequently, these efflux pumps can have an important impact on the intestinal uptake and elimination of many clinically relevant drugs (1, 2). Similarly, ABC transporters at the blood-placenta, blood-testis, and blood-brain barriers protect the corresponding tissue sanctuaries from potentially harmful compounds (1, 2). The subcellular location in various tissues of the ABC transporters discussed in this review is depicted in Figure 1⇓.
Tissue distribution of the human ATP-binding cassette (ABC) transporters ABCB1 (P-gp), ABCG2, and ABCC1-3. A) In the liver, P-gp, ABCG2, and ABCC2 are located in the canalicular (apical) membrane of hepatocytes, pumping their substrates into bile. ABCC3 is present in the sinusoidal (basolateral) membrane of hepatocytes, and pumps its substrates towards the blood circulation. Notably, it was recently shown that Abcc1 is present in activated rat hepatic stellate cells (HSCs), but not in hepatocytes (64). B) In the small intestine, P-gp, ABCG2, and ABCC2 in the luminal (apical) membrane of enterocytes pump their substrates into the intestinal lumen. ABCC1 and ABCC3, located in the basolateral membrane of enterocytes pump their substrates towards the blood circulation. C) In the kidney, P-gp, ABCG2, and ABCC2 are localized in the apical membrane of proximal tubular cells and extrude their substrates into the urine. ABCC1 and ABCC3 are present at the basolateral membrane, pumping their substrates towards the blood circulation. D) At the blood-brain barrier, P-gp, ABCG2, and ABCC2 are located at the apical membrane of capillary endothelial cells, pumping their substrates towards the blood. [ABCC1 (not shown) is also expressed in the basolateral membrane of epithelial cells of the choroid plexus, preventing the entry of its (potentially harmful) substrates into the cerebrospinal fluid (CSF); see (1).] (Tight junctions are indicated by circles.)
ABCC1 is basolaterally located in polarized cells of many tissues. ABCC3 is also a basolateral transporter, predominantly present in liver, gut and kidney, where it transports its substrates towards the systemic circulation (1, 2). For most ABC transporters, transporter-deficient (knockout) mouse models have been generated, and these models have been and still are widely used to study the in vivo functions of efflux pumps; however, given the extensively overlapping substrate specificities of ABC transporters, it is often difficult to unravel their separate roles or understand the significance of functional overlap using single knockout mice. For example, when one transporter is absent, another transporter may partly or completely compensate for its loss. As a consequence, mice in which a single transporter gene is knocked out will often fail to manifest a discrete phenotype. Interestingly, overlapping functions of ABC transporters in vivo were recently observed in a clinical investigation of the protective roles of ABCB1 (P-gp) and ABCG2 (BCRP) in children undergoing chemotherapy (3). This clinical study showed that a combination of two SNP variants of ABCB1 and ABCG2 correlated with increased encephalopathy (brain toxicity), whereas patients with only one of the two variants did not suffer from increased toxicity.
To further study the functional overlap of ABC transporters in vivo, mice deficient in two or more ABC transporters have been systematically generated and characterized (4–14). This review discusses recent findings obtained from combination transporter knockout mice, mainly focusing on pharmacokinetic studies with a number of clinically relevant drugs. These studies illustrate the power of these models in delineating the separate roles of ABC transporters as well as their functional overlap and complementarity.
P-gp–deficient Mice: Abcb1a−/−/Abcb1b−/− Combination Knockout Mice
P-glycoprotein (P-gp/ABCB1) is the first discovered and most extensively studied member of the mammalian ATP binding cassette (ABC) multidrug transporter family (14). P-gp was discovered in cancer cells where it extrudes a wide variety of anticancer drugs. P-gp has a very broad substrate specificity, including bulky amphipathic anticancer drugs such as taxanes, anthracyclines and vinca alkaloids. Consequently, (over)expression of P-gp in tumor cells can lead to multidrug resistance [MDR, (15)]. In addition to cancer cells, P-gp is also widely expressed in normal tissues, including the epithelial cells of intestine and kidneys and the canalicular membrane of hepatocytes, where it pumps its substrates into feces, urine, and bile [Figure 1⇑; reviewed in (1)]. In contrast to the human genome, which encodes P-gp within a single gene (i.e., ABCB1), the mouse genome encodes two isoforms of the protein, within the Abcb1a and Abcb1b genes, and these genes together appear to fulfill the same functions as the single human ABCB1 gene.
Abcb1a−/− mice, initially established to study the in vivo functions of P-gp (16), proved to be a valuable tool for elucidating two important functions of the protein. In particular, Abcb1a (but not Abcb1b) is expressed at the blood-brain barrier and in the intestinal epithelium, and it is consequently situated to restrict the brain penetration and intestinal uptake of harmful compounds (16, 17). Shortly after the generation of Abcb1a−/− mice, mice lacking Abcb1b were generated but failed to display profound biological effects (4). In fact, the concomitant disruption of both Abcb1a and Abcb1b resulted in knockout mice that had normal viability, fertility, and life span, with no apparent physiological abnormalities, despite the complete absence of P-gp (4). These combination knockout mice (Abcb1a −/−/Abcb1b−/−), however, differed from the corresponding single-gene knockout mice; for instance, P-gp substrates accumulated in the bone marrow of the combination knockouts at higher levels than in the marrow of the single-gene knockout mice. In view of the single P-gp–encoding gene in humans, the Abcb1a−/−/ Abcb1b−/− combination knockout mice have become a standard model for investigating the roles of P-gp in physiology, pharmacology, and toxicology [reviewed in (18, 19)].
Lessons from Triple Knockouts: Abcb1a−/−/Abcb1b−/−/Abcc1−/− Mice
ABCC1 (MRP1) is a versatile efflux transporter that can extrude a wide variety of endogenous and exogenous compounds from cells. In contrast to P-gp, which mainly transports non-conjugated neutral or weakly basic lipophilic substrates, ABCC1 is primarily an organic anion transporter, capable of transporting a broad spectrum of drugs conjugated to glutathione, glucuronic acid, and sulfate. In addition, ABCC1 is the major transporter for leukotriene C4 (LTC4), an important endogenous glutathione-conjugated mediator of the inflammatory response (20, 21). Moreover, ABCC1 can also extrude lipophilic and amphipathic xenobiotics such as the anticancer drugs vincristine and etoposide (22–24).
ABCC1 is located basolaterally in polarized cells, although it also occurs in some unpolarized cells, and is expressed in a variety of tissues, including lung, heart, kidney, liver, muscle, colon, testes, bone marrow cells, blood erythrocytes and epithelial cells of the choroid plexus (see also Figure 1⇑). In addition, ABCC1 is found in tumor cells, where it can contribute to multidrug resistance (1). The main function of ABCC1 seems to be the protection of individual cells from accumulation of toxic compounds (1). Two groups have independently generated Abcc1−/− mice (25, 26), and in both instances, the knockout mutants had normal viability, fertility, and life span; however, they were impaired in response to inflammatory stimuli, perhaps reflecting decreased secretion of LTC4 (25). Furthermore, levels of glutathione were elevated in tissues that normally express substantial levels of Abcc1 (26). Abcc1−/− mice were also found to be hypersensitive to the anticancer drugs etoposide and vincristine (25, 26), indicating that Abcc1 plays an important role in drug detoxification. Absence of Abcc1 resulted in increased etoposide-induced damage to the mucosa of the oropharyngeal cavity and to the seminiferous tubules of the testis (27).
Both research groups that had generated Abcc1−/− mice crossed these with Abcb1a−/−/Abcb1b−/− mice (4) to obtain triple knockout (Abcb1a−/−/Abcb1b−/−/Abcc1−/−) mice that were more sensitive to etoposide and vincristine than were the parental strains (4–6), indicating that the chemoprotective effects of P-gp and Abcc1 are additive. Also, cell lines prepared from the triple knockout mice revealed that low-level, endogenous expression of Abcb1a, Abcb1b, and Abcc1 contributes to basal cellular resistance to a range of anticancer drugs (28). Apparently, even non-conspicuous levels of these transporters can affect the innate sensitivity of tumor cells to cancer chemotherapy.
Importantly, studies of the Abcb1a−/−/Abcb1b−/−/Abcc1−/− mice, using etoposide as a probe drug, demonstrate a protective function of ABCC1 in the choroid plexus (5). In Abcb1a−/−/Abcb1b−/− animals, the brain accumulation of etoposide was substantially higher than in their wild-type counterparts, whereas accumulation in Abcc1−/− mouse brain was similar to the wild-type situation. These findings reflect the expression of P-gp by endothelial cells of brain capillaries (Figure 1D⇑), where it pumps substrates back to the blood and limits their entry to the brain. In contrast, Abcc1 is present in the basolateral membrane of choroid plexus epithelial cells. To study a possible role of Abcc1 in reducing accumulation of etoposide in the cerebrospinal fluid, it was first necessary to circumvent the profound role of P-gp in restricting the entry of etoposide to the brain, and for this purpose the use of transporter knockout mice has been crucial. Comparisons of Abcb1a−/−/Abcb1b−/−/Abcc1−/− and Abcb1a−/−/Abcb1b−/− mice revealed that mice deficient for both P-gp and Abcc1 have approximately tenfold higher etoposide concentrations in their cerebrospinal fluid than mice that lack P-gp but express Abcc1. Clearly, the absence of P-gp at the blood-brain barrier allows drug to accumulate in the brains of Abcb1a−/−/Abcb1b−/−/ Abcc1−/− mice; however, mice that retain the Abcc1 gene continue to exclude a significant fraction of drug from the cerebrospinal fluid. The identification of Abcc1 function in vivo is an important finding that was made possible through the judicious use of knockout mice that are deficient in multiple transporters.
Role of ABCG2 and Functional Overlap with P-gp: Abcb1a−/−/Abcb1b−/−/Abcg2−/− Mice
ABCG2 (BCRP) can transport a broad range of endogenous and exogenous substrates and has a substantial overlap in substrate specificity with P-gp (1). Furthermore, in contrast to ABCC1, the tissue distribution of ABCG2 is very similar to that of P-gp, including expression at apical membranes of excretory organs (Figure 1⇑). Consequently, ABCG2 limits the oral availability and tissue penetration of its substrates and mediates their excretion into bile, feces, and urine. In addition, ABCG2 can confer multi-drug resistance to tumor cells. Recent work, relying mainly on the use of Abcg2−/− mice, has further revealed important contributions of ABCG2 to the blood-brain barrier, blood-testis barrier, and blood-fetal barrier (29–32).
Abcb1a−/−/Abcb1b−/−/Abcg2−/− mice were generated by crossing Abcb1a−/−/Abcb1b−/− (4) and Abcg2−/− strains (33). Despite the fact that the resulting mice lack two important apical efflux transporters, they nevertheless demonstrate normal viability (7). The first study employing these mice was conducted to establish the respective contributions of P-gp and ABCG2 to the export of the DNA-binding dye Hoechst 33342, a property that has been used to define the “side-population” phenotype of mammary gland cells and bone marrow cells of mice (7, 34). (Many tissues contain subpopulations of cells defined by the side-population phenotype, which appears to correlate with stem cell–like characteristics.) Both P-gp and ABCG2 had been implicated in Hoechst 33342 export (34, 35). By comparing Abcb1a−/−/Abcb1b−/−, Abcg2−/−, and Abcb1a−/−/Abcb1b−/−/Abcg2−/− mice, it was found that Abcg2 is almost exclusively responsible for the side-population phenotype in bone marrow, whereas both transporters contribute to the side-population phenotype in the mammary gland (7). However, it was recently also shown that some validated mouse mammary stem cells are actually Hoechst 33342 positive and are therefore not likely to be components of the side population (36, 37). The relationship between stem cell functionality and the side-population phenotype thus requires elaboration, and the role of Abcg2 within this relationship likewise awaits further study.
Like normal tissues, cancer cell lines and primary tumor cells also contain subpopulations that manifest the side-population phenotype. This has led to the hypothesis that expression of P-gp and/or ABCG2 in cancer stem cells may render these cells multidrug resistant, which might explain their poor tractability. Although the side-population phenotype may not be a universal stem cell marker, knowledge of the contribution of P-gp and ABCG2 to drug resistance within specific tumor cell populations may prove useful in optimizing cancer chemotherapy. Abcb1a−/−/ Abcb1b−/−/Abcg2−/− mice should be valuable tools to further address these issues.
Like P-gp, ABCG2 is also abundant in the apical membranes of endothelial cells that form the blood-brain barrier (38); however, in contrast to P-gp, the functional role for ABCG2 in this regard had been elusive, despite the availability of Abcg2-deficient mice (33, 39). Some of the confusion may have arisen from experimental design, as most early studies used substrates shared by both P-gp and ABCG2 that failed to define a function based on the knockout of the Abcg2−/− gene alone [reviewed in (29)]. The comparison of Abcb1a−/−/Abcb1b−/−/Abcg2−/− mice with Abcb1a−/−Abcb1b−/− mice, however, revealed a clear function of Abcg2 at the blood-brain barrier. The anticancer drug topotecan, which in vitro is a good ABCG2/Abcg2 substrate and a weaker P-gp substrate, was used to probe transporter function. Compared to wild-type mice, the brain-to-plasma area under the curve (AUC) ratios were not significantly different in Abcg2−/− mice, whereas these ratios were twofold higher in Abcb1a−/−/Abcb1b−/− mice and greater than threefold higher in Abcb1a−/−/Abcb1b−/−/Abcg2−/− mice (40). Although topotecan appears to be a better substrate for ABCG2 than for P-gp in vitro as well as in the mouse intestine (41), P-gp apparently dominates at the brain-blood barrier. Even so, when P-gp is absent, Abcg2 can partly take over the function of P-gp, and when both transporters are absent the brain penetration is markedly increased. This clearly shows that, in addition to P-gp, Abcg2 also restricts the entry of topotecan into the brain. These data further illustrate that in vitro transport data cannot always appropriately predict the relative impact of drug transporters in vivo.
The relative impact of P-gp and Abcg2 is similar on the brain penetration of the tyrosine kinase inhibitors imatinib, lapatinib, and dasatinib (42–44). Although these three compounds are all good in vitro substrates for both P-gp and ABCG2, observation of Abcg2 in restricting brain penetration has been achieved solely by comparing Abcb1a−/−/Abcb1b−/− and Abcb1a−/−/Abcb1b−/−/Abcg2−/− mice; it may be that Abcg2 expression at the blood-brain barrier is significantly lower than that of P-gp. Indeed, it was recently shown in mice of a ddY strain background that P-gp protein levels in brain capillaries are about threefold higher than Abcg2 levels (45). Given that the expression of ABC transporters at the murine blood-brain barrier can differ dramatically between mouse strains (46), it should be noted that the results cited here concerning brain penetration of tyrosine kinase inhibitors (42–44) were obtained in mice of an FVB background. In addition, the RNA expression of Abcb1a in the brain of Abcg2−/− mice may be no different from expression in wild-type brain (44).
Interestingly, examination of human brain capillaries reveals that mRNA levels of ABCG2 are about eightfold higher than P-gp mRNA levels (47), as reflected in seven patients who suffered from brain disease (epilepsy or glioma). Despite possible interference of observations owing to disease, there was more ABCG2 mRNA than ABCB1 mRNA in the microvessels from all patients, regardless of their pathology or treatment (47). Provided that these data reflect protein expression as well as mRNA levels, the evidence may suggest species differences in the relative expression of P-gp and ABCG2 at the brain-blood barrier, and so the contribution of ABCG2 might be more important in human relative to mouse brains.
A Mouse Model for Assessing Hepatic Versus Intestinal Drug Elimination: Abcb1a−/−/Abcb1b−/−/Abcc2−/− Mice
Like P-gp and ABCG2, ABCC2 (MRP2) is expressed at the apical membranes of epithelial cells in kidney and intestine and at the canalicular membrane of hepatocytes (Figure 1⇑). Consequently, ABCC2 plays an important role in the hepatobiliary and renal excretion of its substrates, but in contrast to P-gp and ABCG2, its contribution in restricting uptake of compounds from the intestine seems limited (1). ABCC2 was long regarded primarily as a transporter of organic anionic drugs in vivo, with a preference for substrates conjugated to glutathione, glucuronic acid, and sulfate [reviewed in (24, 48)]. Studies in two rat strains that lack Abcc2 due to spontaneous mutations (i.e., EHBR and TR− strains) and in humans suffering from Dubin-Johnson syndrome (a hereditary deficiency in ABCC2) reveal that this ABC transporter plays an important role in the elimination of bilirubin glucuronides from hepatocytes into the bile (49–51). Recent work has shown that ABCC2 can also transport bulky amphipathic anticancer drugs in vitro and in vivo (8, 52), and ABCC2 thus has a substantial overlap in substrate specificity with P-gp.
Recently, we and others independently generated Abcc2−/− mice (52, 53). We crossed our Abcc2−/− mice with Abcb1a−/−/Abcb1b−/− mice (4) to obtain Abcb1a−/−/Abcb1b−/−/Abcc2 −/− mice (8, 52). Similar to the parental Abcc2−/− mice, the Abcb1a−/−/Abcb1b−/−/ Abcc2−/− mice had a ~25% increased liver weight, and the bile flow was reduced by ~50% due to absence of Abcc2-mediated biliary glutathione excretion (52). Furthermore, as a consequence of reduced excretion (52), the Abcc2-deficient mice manifested plasma concentrations of conjugated bilirubin concentrations that were elevated threefold relative to wild-type mice (8). Overall, Abcb1a−/−/Abcb1b−/−/Abcc2−/− mice appear in many respects very similar to Abcc2−/− mice and are likely as amenable to pharmacological analysis.
We used the Abcb1a−/−/Abcb1b−/−/Abcc2−/− mice and the corresponding single knockout mice to study the separate and combined impact of P-gp and Abcc2 on the elimination of the lipophilic amphipathic anticancer drugs doxorubicin (52) and paclitaxel (8). The hepatobiliary excretion of doxorubicin was mainly dependent on P-gp, with a modest role for Abcc2 (52). Surprisingly, the excretion of paclitaxel into the bile was by contrast dominated by Abcc2, with only a minor contribution of P-gp (8), despite the fact that paclitaxel is an excellent P-gp substrate in vivo and that its oral availability is largely restricted by P-gp (8, 17). The abrogated biliary excretion of paclitaxel in Abcc2−/− mice results in a 1.3-fold higher AUC compared to wild-type upon intravenous paclitaxel administration. Interestingly, the AUCiv for paclitaxel in Abcb1a−/−/Abcb1b−/− mice was also 1.3-fold increased. This could be explained by the dominant role of P-gp in the gut, where it mediates the direct intestinal excretion of paclitaxel from the blood across the intestinal wall into the gut lumen (8, 17). Very likely, it also restricts the intestinal reuptake of paclitaxel after hepatobiliary secretion of the drug in the gut. Absence of both transporters results in an additive 1.7-fold higher AUCiv in Abcb1a−/−/Abcb1b−/−/Abcc2−/− mice [reviewed in (54)]. These studies demonstrate the power of the combination knockout mice to elucidate the tissue-specific contribution of P-gp and ABCC2 functions as well as the separate and combined impact of these transporters on the elimination of lipophilic amphipathic drugs.
Complementary Functions of ABCC2 and ABCC3 in the Liver: Lessons from theAbcc2−/−/Abcc3−/− Mouse
The ABC transporters ABCC2 (MRP2) and ABCC3 (MRP3) are both members of the multidrug resistance-associated protein (MRP/ABCC) family, and they have similar structures and partially overlapping substrate specificities (24). They can both transport a range of physiological substrates, such as bilirubin glucuronides, estradiol-17 -glucuronide, and some bile salts (24). Furthermore, ABCC2 and ABCC3 can transport many drugs, such as epipodophyllotoxins and methotrexate, as well as a range of drug conjugates, in particular, drug glucuronides such as morphine glucuronide (11, 24). The general tissue distribution of ABCC3 is quite similar to that of ABCC2 (see Figure 1⇑); however, in contrast to the apical localization of ABCC2, ABCC3 is expressed baso-laterally, pumping its substrates towards the circulation (1, 24). Interestingly, deficiency of the Abcc2 transporter in mice, rats, and humans results in significantly increased Abcc3 protein expression in the liver. Thus, with reduced biliary output in the absence of Abcc2 (52, 55, 56), Abcc3 may act, in a compensatory mechanism, to transport substrates into the circulation for urinary excretion. Note that single Abcc3−/− mice show very few abnormalities (48).
Recently, Abcc2−/−/Abcc3−/− mice have been generated, both in C57BL/6 and FVB backgrounds (11–13), and they have been used to study the overlapping physiological and pharmacological functions of both transporters in vivo. Physiological characterization of Abcc2−/−/Abcc3−/− mice shows normal life spans and body weights, although liver weights are significantly (36–49%) increased compared to wild-type mice (12, 13); the livers otherwise are normal, both macroscopically and microscopically (12). It could be that accumulation of some shared Abcc2 and Abcc3 substrates in the liver induces liver proliferation. Furthermore, bile flow in Abcc2−/−/ Abcc3−/− mice is significantly decreased compared to wild type (12, 13), as shown previously for Abcc2−/− mice (52, 53). Previously it had been hypothesized that increased Abcc3 protein in the liver of Abcc2-deficient rats, mice, and humans would result in increased plasma levels and urinary excretion of conjugated bilirubin (52, 55). Analysis of bilirubin concentrations in the plasma, bile, and urine of Abcc2−/−/Abcc3−/− mice indeed confirms that Abcc3 is necessary for the increased sinusoidal efflux and urinary excretion of bilirubin glucuronides (12). Additionally, similar observations of Abcc2−/−/Abcc3−/− mice suggest that the two transporters function to coordinate the elimination of methotrexate (12), 7-hydroxymetho-trexate (12), morphine-3-glucuronide (11), and fexofenadine (13). Administration of morphine to methotrexate-treated mice significantly reduces plasma clearance of methotrexate (57), which is of clinical interest because morphine and methotrexate are often co-administered in cancer treatment. The results obtained with the Abcc2−/−/Abcc3−/− mice described here suggest that these effects could be caused by competition between methotrexate and morphine-3-glucuronide for elimination via Abcc2 and Abcc3. These observations have obvious clinical implications for patients with reduced expression or activity of ABCC2 and/or ABCC3 and to Dubin-Johnson patients (58).
Functional Overlap of ABCG2 and ABCC2 in Hepatobiliary Excretion: TheAbcc2−/−/Abcg2−/− Mouse Model
As described above, the tissue distribution and polarized (apical) localization of ABCC2 and ABCG2 are quite similar (Figure 1⇑), and the substrate specificities of ABCC2 and ABCG2 are broad and substantially overlapping as well. Both proteins can transport many drugs, including anti-cancer drugs like methotrexate, doxorubicin, and SN-38, as well as dietary toxins [e.g., the carcinogen PhIP (2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine)], and a range of glucuronide and sulphate conjugates of endogenous and exogenous compounds (1, 59, 60). Given the overlap in substrate specificity and tissue distribution, the functions of ABCC2 and ABCG2 as assessed with single knockout mice could not be definitive. We have therefore generated Abcc2−/−/Abcg2−/− mice in a well-characterized FVB background (61). Like the other ABC transporter combination knockout mice generated so far, these mice are viable, and they recapitulate the phenotypes of the single knockout mice, such as the hypersensitivity to the phototoxic dietary compound pheophorbide a, reminiscent of Abcg2−/− mice, and the conjugated hyperbilirubinemia of Abcc2−/− mice (33, 52). This suggests that the physiological functions of ABCC2 and ABCG2 are not overlapping, or may still be taken over by other systems such as enzymes or other transporters.
Because wild-type mice of an FVB background, in contrast to some other genetic backgrounds, do not have Abcc2 protein in brain capillary endothelial cells (47), the Abcc2−/−/Abcg2−/− mice we have generated cannot be used to investigate overlapping functions of Abcc2 and Abcg2 in the blood-brain barrier. However, we did use these mice to investigate the effect of Abcc2 and Abcg2 on the disposition of methotrexate and its primary metabolite 7-hydroxymethotrexate in vivo (61). We found that Abcc2 and Abcg2 have additive effects on the plasma elimination of methotrexate, which reflects the impact on the biliary excretion of the drug. Whereas substantial biliary excretion persists in both single knockout strains, the double knockouts are severely compromised in this respect, showing that Abcc2 and Abcg2 in mice are the main transporters for the excretion of methotrexate and 7-hydroxymethotrexate into the bile. Interestingly, in Abcg2−/− mice, we found no differences in the plasma concentration-versus-time curves for 7-hydroxymethotrexate; however, compared to Abcc2−/− mice, an additional effect of Abcg2 deficiency on the plasma concentrations of 7-hydroxymethotrexate is observed in Abcc2−/−/Abcg2−/− mice, indicating that when Abcc2 is absent, Abcg2 can partly compensate for its loss. We expect that Abcc2−/−/Abcg2−/− mice will be extensively used to determine the in vivo effects on pharmacokinetics of drugs that are substrates of both transporters.
Conclusions
In the past few years, a large set of ABC transporter combination knockout mice has been generated and used for the analysis of overlapping and complementary transport functions in vivo (Table 1⇓). These mice will help investigators determine the consequences of reduced expression or activity of ABC transporters in patients treated with potentially toxic drugs. Furthermore, as ABC transporters can also transport endogenous compounds and food-derived toxins (e.g., carcinogens), these mice can be used to investigate the relative effects of ABC transporters on normal health. For example, ABC transporter combination knockout mice may be used to study whether loss of functional activity of one or more ABC transporters can influence risks for cancer. Studies on the overlapping or complementary effects of ABC transporters in multidrug resistance in vivo will also be facilitated through the use of these new mouse strains.
ABC Transporter Functions as Determined from Combination Knockout Mice
The results obtained so far have demonstrated that the relative effect of each ABC transporter on drug pharmacokinetics can be highly dependent on the substrate, administration route, and the tissue or organ under investigation. Very likely, dosage also determines which ABC transporter will be more important for the pharmacokinetics of any drug. As illustrated in this review, it is often difficult to use only in vitro assays to accurately predict the in vivo effects of ABC transporters, and combination knockout mice are therefore invaluable tools for these types of studies. To date, the concerted deletion of multiple ABC transporters has been limited to double-knockout combinations, although we have also discussed animals that lack three different ABC transporters. Because various drugs and toxins, as well as their metabolites, can be transported by more than two ABC transporters, there is an obvious need to extend the current set of models to triple-, quadruple- or even higher order–combinations of knockouts. Furthermore, crossing ABC transporter knockout mice with knockout models of other drug elimination mechanisms, such as drug-metabolizing enzymes, will give even more insight into the interplay between these different systems in vivo.
Of course, as ABC transporters are certainly involved in protecting the organism from endogenous and exogenous toxins, it will be interesting to see how many additional ABC transporter genes can be deleted without incurring lethality. Besides increasing fundamental knowledge on ABC transporter function, this is of interest because the inhibition of one or more ABC transporters is a clinical strategy (62, 63). We have recently even been able to generate Abcb1a−/−/ Abcb1b−/−/Abcc2−/−/Abcg2−/− and Abcc2−/−/Abcc3−/−/ Abcg2−/− mice which are viable, fertile and have normal life spans (unpublished results). This suggests that the physiological functions of these proteins are not essential, at least not in the protective environment of the lab. In the near future, these strains can be used for pharmacological analyses. Further investigation of the generated ABC transporter knockout mice will likely reveal additional physiological and pharmacological functions of ABC transporters, along with related therapeutic opportunities.
Acknowledgments
We thank Dr. Piet Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands) for critical reading of the manuscript. This work was supported in part by grant NKI 2003–2940 from the Dutch Cancer Society.
- © American Society for Pharmacology and Experimental Theraputics 2009
References

Alfred H. Schinkel, PhD, is a group leader at the Division of Molecular Biology of the Netherlands Cancer Institute in Amsterdam, The Netherlands. His research focuses on the pharmacological, physiological and toxicological roles of multidrug efflux and uptake transporters, and of the multispecific Cytochrome P450 3A drug-metabolizing enzymes. Address correspondence to AHS. E-mail a.schinkel{at}nki.nl; fax +31 20 6691383.

Maria L.H. Vlaming, PhD, is currently working as a project leader at TNO Quality of Life in Zeist, The Netherlands. Her research focuses on the pharmacological functions of transporters in vitro and in vivo. She worked as a PhD student in the Laboratory of Dr. Alfred Schinkel at The Netherlands Cancer Institute in Amsterdam on the generation and characterization of ABC transporter combination knockout mice.

Jurjen S. Lagas, PhD, is currently working as a pharmacist at the Slotervaart Hospital in Amsterdam, The Netherlands. He worked as a PhD student in the Laboratory of Dr. Alfred Schinkel at the Netherlands Cancer Institute in Amsterdam on pharmacokinetic assessment of ABC multidrug transporters, using ABC transporter combination knockout mice.