
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
Regenerative Pharmacology for Diabetes Mellitus
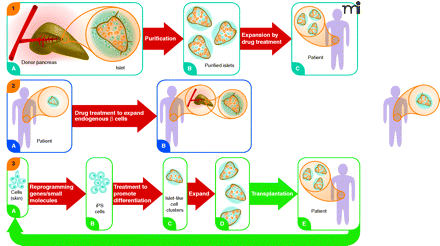
Three regenerative pharmacology strategies for diabetes treatment. Strategy 1. From donated pancreas Islets of Langerhans (A) are purified (B) and incubated with a small molecule drug that stimulates proliferation of β cells (colored orange; non-β cells in blue). Treated islets with enhanced β-cell mass (C) are transplanted into a recipient with diabetes. Patient requires immunosuppressive drugs to avoid transplant rejection. Strategy 2. Patient with diabetes has fewer β cells in shrunken islets (A). Treatment with a drug that stimulates proliferation of these endogenous cells expands the β-cell mass without need for islet transplantation (B). Strategy 3. Cells from a patient with diabetes are obtained from a minimally invasive biopsy—e.g., skin, hair, or peripheral blood—and grown briefly in culture (A). Induced pluripotent stem (iPS) cells are obtained by reprogramming the cells using a small set of pluripotency genes and/or small molecules (B). The iPS cells are differentiated in vitro to pancreatic islet-like clusters (ILCs) containing β cells using known biological factors and/or small molecules (C). The β cells in these structures may be further expanded [(D)], as in Strategy 1, and then transplanted back into the patient (E). The ILCs are transplanted into the patient. Immunosuppressive therapy is not required to prevent rejection. The strategy also could be used beginning with embryonic stem (ES) cells from an unrelated individual. In this case patients would require immunosuppressive drugs. None of these strategies addresses underlying causes of diabetes, such as autoimmunity (T1D) or insulin resistance (T2D), which must be addressed by additional treatments. However, increased functional β-cell mass would significantly improve regulation of blood sugar. Broad red arrows indicate points of possible pharmacological intervention.


