
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
PHD Fingers: Epigenetic Effectors and Potential Drug Targets
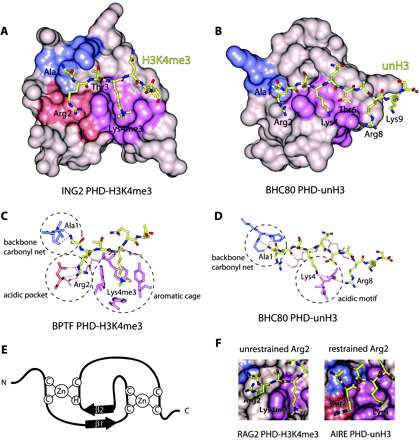
Figure 2
Structural bases of histone code interpretation by PHD fingers. The crystal structures of (A) H3K4me3-bound ING2 PHD and (B) unH3-bound BHC80 PHD. The binding pockets for Ala1, Arg2, and Lys4me3/Lys4 are colored blue, salmon, and purple, respectively. The detailed coordination of these residues is highlighted (dashed circles) for (C) BPTF PHD-H3K4me3 and (D) BHC80 PHD-unH3. E) The PHD finger fold is characterized by the canonical Cys4-His-Cys3 (C4HC3) sequence that coordinates two zinc ions and contributes to the typical two-stranded anti-parallel β sheet structure. F) The dichotomy of coordination of Arg2 in the RAG2 PHD-H3K4me3 complex (left) and AIRE PHD-unH3 complex (right).


