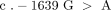Pharmacogenetics of Anticoagulants
- Anders Rane anders.rane{at}ki.se1,2
- Jonatan D. Lindh jonatan.lindh{at}ki.se1
Abstract
Warfarin, acenocoumarol, and phenprocoumon are among the major anticoagulant drugs worldwide. Because of their low therapeutic index and serious adverse reactions (ADRs), their wide use, and their varying kinetics and pharmacogenetic dependence, it is of great importance to explore further possibilities to forecast the dose beyond conventional INR measurements. Here, we describe particulars of the relative pharmacogenetic influence on the kinetics of these agents, the population distribution of genetics risk groups, and novel data on clinical features with influence on dose requirement and ADR risk. The usefulness of genetic information prior to and soon after start of therapy is also discussed. The current renewed focus on these issues is caused not only because of new genetic knowledge and genotyping facilities but also because of the high rate of serious ADRs. Application of these measures in the care of patients with anticoagulant therapy is important awaiting new therapeutic principles to be introduced, which may take long time still.
1. Introduction
Warfarin, phenprocoumon, and acenocoumarol are the most used anticoagulants worldwide. It is a paradox that at the same time these drugs are amongst the top 5 drugs all categories causing most serious and fatal events. Their therapeutic index is very low. The major reason is the difficulty to tailor the dose according to the individual dose requirement which varies widely between patients. Few other drugs than warfarin have a broader therapeutic dose window, 20–30-fold, possibly with exception for narcotic analgesics.
There are a number of determinants of anticoagulant dose requirement. Historically, it has been known for long that a vitamin K containing diet has an influence on the efficacy of warfarin and other coumarins. It has also been known for a long time that increasing age reduces the dose needed to achieve a therapeutic anticoagulant effect through inhibition of coagulation factor synthesis [1]. Other factors that are known to influence dose requirement include concurrent medication, and body surface area [2–4].
2. Anticoagulation-Related Haemorrhage
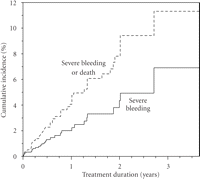
We embarked upon a prospective study of patients in need of anticoagulation because of atrial fibrillation, deep venous thrombosis, pulmonary emboli, and so forth [5]. In our prospective study, warfarin naïve patients consented to donate a blood sample for genotyping at the start of warfarin treatment. The study included 1523 patients. All of them were monitored according to local clinical routines at 40 different study sites in Sweden. Warfarin bleedings defined according to WHO definition of severe adverse drug reaction [6] as well as other adverse reactions were reported to the study centre where the blood samples were stored in a biobank sample collection. In this cohort of patients starting treatment with warfarin, the incidence of first-time severe haemorrhage was 2.3 per 100 patient years (95% confidence interval 1.4–3.1). During a mean followup time of 10 months, 1.8% of the included patients died and the overall incidence of severe bleeding or death (all-cause) was 4.3 per 100 patient years (95% c.i. 3.2–5.5) (Figure 1). Our assessment of the incidence was lower than has been reported earlier [7–9], probably because of the more strict criteria stipulated by WHO and applied by us.
Cumulative incidence of severe bleeding and the composite of severe bleeding and all-cause mortality in the warfarin-treated WARG cohort [5].
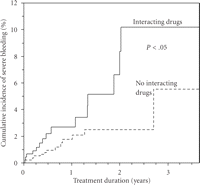
Although the incidence of bleeding was lower than that seen in many older studies, it is evident that haemorrhagic complications remain a major clinical problem in anticoagulation with vitamin K antagonists. When investigating potential predictors of severe bleeding in a multivariable model (Table 1), we identified two factors significantly associated with this adverse outcome—male gender (HR = 2.8, 95% c.i. 1.1–7.3) and use of drugs potentially interacting pharmacologically with warfarin (HR = 2.3, 95% c.i. 1.1–4.9). The vastly increased risk in men was an unexpected finding and previous studies have oppositely showed an increased risk in female patients [8, 10, 11]. Although the association between the use of interacting drugs and bleeding may have been anticipated, the evidence of an overall effect of the full spectrum of interacting drugs has been scarce prior to our findings. The association remained even after several years of treatment (Figure 2) indicating prevailing difficulties in managing the effects of drug interactions in warfarin-treated patients.
Predictors of severe bleeding in the warfarin-treated WARG cohort [5].
Cumulative incidence or severe bleeding in warfarin-treated users and non-users of drugs potentially interacting with warfarin. Data from the WARG cohort [5].
3. Pharmacogenetics of Anticoagulants
With the development of pharmacogenetics, the interest was focused on enzymes catalysing the metabolism of anticoagulants to see if there was a relation between dose requirement and genetic polymorphisms in these genes.
Early studies [12, 13] demonstrated an important role of CYP2C9 in the metabolism of warfarin, in particular the 7-hydroxylation of the more active S-enantiomer.
Aithal et al. were among the first to show a clinical implication of the CYP2C9 polymorphism. They studied hospitalised patients and found that low-dose requirement was associated with CYP2C9∗2 and CYP2C9∗3 alleles [14]. These findings have subsequently been confirmed in numerous clinical or experimental study settings [4, 15–18].
Many polymorphisms of CYP2C9 have been described. The common CYP2C9∗2 and ∗3 allelic functional variants have a compromised ability to catalyze the oxidation of S-warfarin, with in vitro activities of only 12 and 5%, respectively, for the homozygous mutant forms [19, 20]. Other enzyme members of the CYP2C family have some catalyzing capacity albeit very little and of minor importance for the clearance of S-warfarin.
The identification and localisation of the VKORC1 gene [21, 22] paved the way to studies that demonstrated a profound dependence of warfarin dose on the polymorphic VKORC1 gene [23–26]. Since warfarin targets the Vitamin K epoxide reductase, it is conceivable that any functional mutations in this gene will perturb the balance between the influence of warfarin and vitamin K on the enzyme activity. There are a number of such SNPs with strong linkage disequilibirium. One of these SNPs is located in the promoter region and usually included in studies of the VKORC1 genetics and warfarin dose requirements, namely −1639G > A (rs 9923231) [25]. The A allele is associated with the need of lower doses than the G allele. The association is such that a homozygous carrier of the A allele requires a warfarin dose approximately 50% of that of an individual that is homozygous for the G allele [27]. Similar effects have been ascribed to the VKORC 1173C > T (rs9934438) polymorphism. However, these two SNPs are in pronounced linkage disequilibrium and are interchangeably used as tag SNPs for differentiation between a low-dose haplotype A (−1639A and 1173T) and a high-dose haplotype B (−1639G and 1173C) [25]. Other SNPs have been independently associated with altered warfarin dose requirements, but their contribution to the interindividual variability is small compared to the −1639G > A and 1173C > T polymorphisms [28, 29]. Below, the haplotype A/B nomenclature will be used to denote VKORC1 polymorphisms.
Candidate gene work with univariate regression analysis of small samples has limited power to detect warfarin dose association with allelic variants with weak influence [30]. However, multivariate regression improves this possibility. Thus, both CYP2C9∗3 and ∗2 were statistically significant predictors of warfarin dose when data were corrected for nongenetic and other dose predictors [30].
Genome-wide association studies (GWASs) are increasingly applied within pharmacogenomics and in studies of multiple gene factors
behind certain diseases. This approach was used by us in 1053 of 1523 patients of the WARG cohort [31] and was sufficiently powered to detect a genome-wide significance of 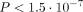 for polymorphisms with modest influence on therapeutic dose need. Univariate regression analysis of each SNP analysed (
for polymorphisms with modest influence on therapeutic dose need. Univariate regression analysis of each SNP analysed (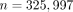 ) disclosed just two important loci/SNP regions. They were in the VKORC1 and CYP2C9 genes. However, multivariate regression
analysis also revealed another SNP although with weaker association. This SNP is located in a gene encoding a cytochrome P450
enzyme protein, CYP4F2, a putative catalyst of Vitamin K1 oxidation [32].
) disclosed just two important loci/SNP regions. They were in the VKORC1 and CYP2C9 genes. However, multivariate regression
analysis also revealed another SNP although with weaker association. This SNP is located in a gene encoding a cytochrome P450
enzyme protein, CYP4F2, a putative catalyst of Vitamin K1 oxidation [32].
4. Translation into Clinically Useful Algorithms
The pharmacogenetic knowledge about warfarin pharmacokinetics and pharmacodynamics has to be translated into useful dosing guidelines in order to be accepted in the clinical care of patients in need of warfarin. Several algorithms have been suggested on the basis of patient information with or without genetic data on the VKORC1 and CYP2C9 polymorphisms. By now, there is unequivocal evidence that combined clinical and genetic information generates more accurate dose prediction than non-genetic information alone [33, 34].
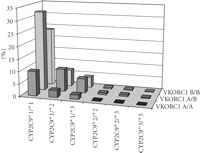
Considering the allele frequencies of CYP2C9 and VKORC1, it is obvious that about 3/4 of the Caucasian population carry at least one allelic variant in these genes with lower warfarin dose requirement (Figure 3). Table 2 demonstrates the fractional distribution of different haplotypes based on the allele frequencies given for each of the genes.
Combined CYP2C9/VKORC1 genotype frequencies calculated from known allele frequencies in Caucasians.
Combined CYP2C9 and VKORC1 genotype frequencies. The striped bar (1/4 of the Caucasian population) indicates individuals without any variant alleles (CYP2C9∗2, CYP2C9∗3 or VKORC1 A), while the solid bars represent genotypes with one or more variant alleles (3/4 of the population).
When based on a multiple regression model that was developed in the WARG material [4], the algorithm for estimation of warfarin dose (Table 3) predicts a 12-fold difference between the extreme haplotypes in the upper left and lower right corners of Table 2. According to our algorithm (Table 3), this difference increases to 14-fold if the patients are treated with three interacting drugs, demonstrating the complexity of interactions between all predictors of the warfarin dose. This is because the dose requirement decreases much less (−5%) for the CYP2C9∗1/∗1, VKORC1B/B combination than for the CYP2C9∗3/∗3, VKORC1A/A genotype (−18%).
The WARG warfarin dose algorithm [4].
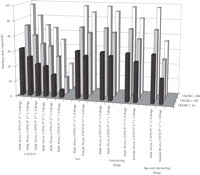
Figure 4 demonstrates the effect of genotype, sex, interacting drugs, and age, alone or in different combinations, on warfarin dose requirement. This variation has significant clinical implications. It is obvious from figure 4 that any genetic variation in the two genes compared to CYP2C9∗1/∗1, VKORC1B/B, all other parameters being equal, causes more than 15–20% decrease in dose requirement. This feature corresponds to about 3/4 of the population. The conspicuous effect on dose requirement of common patient characteristics is also shown, for example, 80 versus 50 years of age, three interacting drugs versus none, and so forth.
Warfarin dose predictions based on the patient's sex, age, CYP2C9 and VKORC1 genotype, and use of interacting drugs. “Drugs” refer to the number of concomitantly used drugs potentially interacting with warfarin, causing an increased INR (drugs lowering INR are not included in the algorithm).
In the clinical setting, it is common that warfarin treatment has to be commenced without genetic information about the patient. Such information, if requested, is usually not provided until the patient has already received 5–10 warfarin doses. The value of genetic information is expected to decrease the later it is available and after one week the information is considered irrelevant by some investigators. However, data generated in a broad warfarin consortium have demonstrated an additive informative value of genetic data on VKORC1 and CYP2C9 even when it is obtained 7–9 days after the start of treatment [35]. Previous and ongoing studies have found that after 4–6, 7–9, and 14 days of INR-guided therapy, genotyping still improves the prediction of maintenance dose by 4–15%, 2–14%, and 4%, respectively [35–39]. Individual warfarin dose predictions based on VKORC1 and CYP2C9 genotype and clinical factors (with or without INR data) are available, for example, at the nonprofit website http://www.warfarindosing.org/.
5. Determinants of Phenprocoumon and Acenocoumarol Maintenance Dose Requirements
Other coumarin derivates such as phenprocoumon (PC) and acenocoumarol (AC) are widely prescribed in continental European countries and Latin America, whereas warfarin is the drug of choice in US, UK, and the Scandinavian countries. There are major differences in the pharmacokinetics of the coumarin-based anticoagulants. Thus, acenocoumarol has a much shorter half-life (2–8 hours) as compared to warfarin (30–45 hours) or phenprocoumon (156–172 hours) [40]. The genetic contribution to variation in kinetics and dose requirement is more pronounced for warfarin than for acenocoumarol or phenprocoumon. On the other hand, adjustment of warfarin dose is easier than that of phenprocoumon because of its shorter half-life and short time to achieve steady state.
CYP3A4 and CYP2C9 are the major catalysts of the phenprocoumon 7-hydroxylation, and therefore the polymorphic CYP2C9 enzyme contributes less to the metabolism of phenprocoumon compared to warfarin [40, 41]. In addition, a part of the drug is excreted directly via the kidneys or the bile. Because of this difference, phenprocoumon is sometimes preferred as an alternative in patients harboring the CYP2C9∗2 or CYP2C9∗3 alleles which significantly compromise the metabolism of warfarin, and to a high degree also acenocoumarol [42, 43].
Identification of the vitamin K epoxide reductase C1 gene [21] paved the way to the identification of functional mutations in VKORC1 gene, both those conferring warfarin resistance [22] and those causing an increase in warfarin dose requirement [25]. A recent publication [44] compared the relation between on one hand the VKORC1 and CYP2C9 genotype, and on the other, the mean weekly dose requirement for acenocoumarol and phenprocoumon. The difference in weekly average dose for individuals carrying VKORC1 AA and BB genotypes was approximately two-fold (as for warfarin) for each of the drugs, with the highest doses in the BB group. The average reduction in acenocoumarol and phenprocoumon dose requirements in patients with mutant variants of CYP2C9 included in their study was at most 1/3 of the average dose in wild type individuals. These results clearly demonstrate the difference in dependence on the CYP2C9 genetic polymorphisms of these drugs as compared to warfarin. Other genes of relevance for warfarin disposition or vitamin K action were not found to have any impact on the dose requirement of acenocoumarol and phenprocoumon [44].
Although phenprocoumon is less dependent on CYP2C9 for its elimination compared to acenocoumarol, polymorphisms in CYP2C9 have been associated with an increased risk of overanticoagulation and bleedings for both drugs [45–48]. There is still limited information regarding the potential impact of VKORC1 polymorphisms on the risk of adverse outcomes in patients treated with acenocoumarol or phenprocoumon. Nevertheless, the VKORC1 A haplotype has been associated with an increased risk of overanticoagulation (INR > 4) in patients treated with acenocoumarol and with major bleeding in phenprocoumon-treated individuals [49, 50].
In summary, the clinical use of coumarins is associated with great risk of over- or underanticoagulation. The risk of severe adverse reactions including fatal outcome is among the highest known in drug therapy. It has been manageable by virtue of INR monitoring but needs to be further reduced. The new knowledge about genes important for the metabolism and effect of these drugs suggest genotyping to be applied in the clinic since the future development of anticoagulant therapy is uncertain even if new therapeutic principles are on the way to be introduced. Ongoing large clinical trials such as COAG (Clarification of Optimal Anticoagulation Through Genetics) and EU-PACT (The European Pharmacogenetics of Anticoagulant Therapy trial) will further elucidate these important issues and it remains to be seen if their results will modify our current opinion [51, 52].
Acknowledgments
Part of this work was supported by the Swedish Science Council (04496) and Nycomed AB (Sweden).
- Received June 10, 2010.
- Accepted August 17, 2010.
- © 2010 Anders Rane and Jonatan D. Lindh.