
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
γ-Secretase: A Catalyst of Alzheimer Disease and Signal Transduction
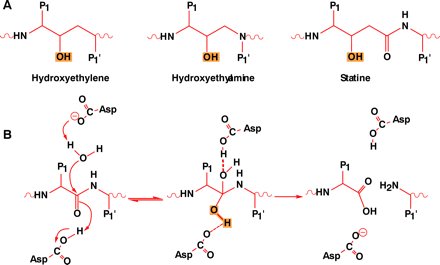
Figure 5.
The design of inhibitors of aspartyl proteases.
A. Isosteres that are commonly used to design transition-state analogs of proeolysis. P1 and P1' represent substituents that mimic the amino acid side chains of natural substrates.
B. The reaction mechanism of aspartyl proteases indicating the two aspartates involved in catalysis. An activated water molecule is required for proteolysis. P1 and P1' represent amino acid side chains. The hydroxyl character of the tetrahydryl transitionstate that is mimicked by the isosteres is highlighted.


