
- Institution: Stanford Univ Med Ctr Lane Med Lib/Periodical Dept/Rm L109
- Sign In as Member / Individual
The GoLoco Motif: Heralding a New Tango Between G Protein Signaling and Cell Division
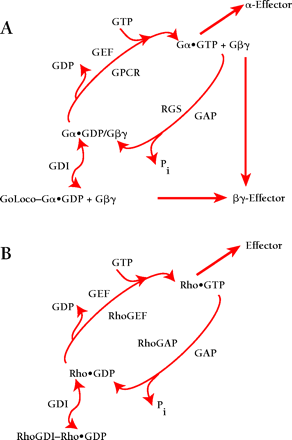
Revised model of heterotrimeric G protein signaling. A. In comparison to the standard model shown in Box 1, this revised model introduces GoLoco proteins, as guanine nucleotide dissociation inhibitors (GDIs), into the traditional array of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) for Gα subunits (i.e., GPCR and RGS proteins, respectively). GoLoco binding to Gα•GDP results in the release of Gβγ independent of receptor-mediated GEF activity, thus allowing Gβγ to activate downstream effector pathways. B. By way of comparison, the current model of signaling by the small GTPase, Rho, involves GDI, GEF, and GAP activities in addition to effectors stimulated by Rho•GTP. However, the RhoGDI–Rho•GDP complex is thought to sequester Rho from membrane-delimited activation, and there is currently no evidence that this complex directly activates downstream signaling (48).


