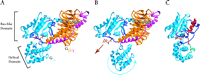The GoLoco Motif: Heralding a New Tango Between G Protein Signaling and Cell Division
- Department of Pharmacology Lineberger Comprehensive Cancer Center Unc Neuroscience Center The University of North Carolina at Chapel Hill Chapel Hill NC 27599-7365 USA
- DPS. E-mail dsiderov{at}med.unc.edu; fax 919-966-5640.
Abstract
The Gα and Gβγ components of heterotrimeric G proteins, typically associated with cell-surface receptor signaling, also partake in the macromolecular interactions that underlie cell polarity and cell division. Proteins with Gα-binding GoLoco motifs, such as Drosophila melanogaster Pins (for Partner of Inscuteable) and its mammalian counterpart LGN, participate in multi-protein complexes that maintain cellular asymmetry and orderly segregation of chromosomal content and daughter cell bodies. The GoLoco motif was recently identified as a selective Gα-binding partner: the GoLoco–Gα interaction can displace Gβγ and inhibit guanine nucleotide release from the bound Gα subunit. Recent x-ray crystallographic studies suggest ways in which GoLoco-motif peptides may modulate heterotrimeric G protein signaling. Such peptides could be exploited to help dissect the signals that underpin cell polarity and cell division processes.
Heterotrimeric G proteins regulate signaling pathways downstream of plasma membrane-bound receptors. Several mechanisms have
been discovered that promote the separate effector functions of the Gα and the Gβγ subunits. Recent efforts have identified GoLoco motif-containing proteins as Gα-associated proteins that can regulate the reassociation of Gα to Gβγ, and that might effect signal transduction on their own. In what physiological processes are these proteins involved?
INTRODUCTION
The processing of cellular signals is accomplished by a complex network of proteins, lipids, ions, and small molecules. Signals can be propagated from cell to cell by the action of hormones released from the same (autocrine), neighboring (paracrine), or distant cells (endocrine). Signals can also result from neurotransmitter release at the synaptic cleft or can be kept internal to an individual cell. In all of these cases, information is transmitted through the action of multiple signaling molecules, some of which are proteins that alternate between an “on” and an “off” state. Guanine nucleotide binding proteins, or “G proteins,” are among the most ubiquitous of these cellular switches and alternate between a guanosine diphosphate- (GDP)-bound off state and a guanosine triphosphate- (GTP)-bound on state.
The standard model of G protein–coupled receptor (GPCR) signaling is detailed in Box 1. The Gβγ heterodimer couples Gα to the receptor and inhibits its release of GDP; thus, Gβγ possesses a type of guanine nucleotide dissociation inhibitor (GDI) activity. Ligand-occupied GPCRs stimulate signal onset by acting as guanine-nucleotide exchange factors (GEFs) for Gα subunits, facilitating GDP release, subsequent binding of GTP, and release of the Gβγ dimer. Effector interactions with the GTP-bound Gα and free Gβγ subunits propagate the signal forward. Regulators of G protein signaling (RGS) accelerate the GTPase activity of Gα subunits and stimulate signal termination by promoting the reformation of the Gαβγ heterotrimer. Thus, the standard model of GPCR signaling assumes that the Gα subunit's lifetime in the GTP-bound state controls the duration of signaling from both Gα•GTP and the free Gβγ subunit. Recently, this assumption has been challenged by the discovery of an additional class of Gα-regulatory proteins that contain characteristic “GoLoco” motifs with which they specifically bind to Gα•GDP subunits. This GoLoco–Gα•GDP interaction excludes Gβγ binding, and thus is capable of permitting continued Gβγ-effector signaling in the absence of receptor-catalyzed Gα•GTP formation. More surprisingly, GoLoco-motif proteins have recently been implicated in the establishment of cell polarity, asymmetric cell division, spindle-pole organization, and chromosomal segregation. Here, we review the current literature regarding the structure and function of this novel G protein regulatory motif, including our recent solution of an atomic-resolution structure of a GoLoco–Gα•GDP complex, and speculate as to the potential use of GoLoco-motif peptides as novel pharmacological tools for further interrogation of G protein signaling pathways both at the plasma membrane and in the nucleus.
Standard model of the GDP-GTP cycle governing activation of heterotrimeric G protein–coupled receptor (GPCR) signaling pathways.
In the standard model of heterotrimeric G protein signaling, seven-transmembrane–domain G protein coupled–receptors (GPCRs) are intimately associated with a membrane-bound heterotrimer composed of Gα, Gβ, and Gγ subunits. Gβ and Gγ form an obligate heterodimer that binds tightly to GDP-bound Gα subunits, facilitates Gα localization to the plasma membrane (51), enhances its functional coupling to receptor, and inhibits the release of GDP from Gα [i.e., Gβγ dimers exhibit guanine-nucleotide dissociation inhibitor (GDI) activity (52,,53)].
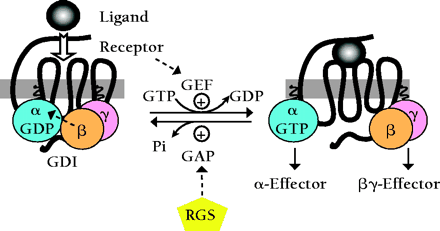
The binding of extracellular ligand to the GPCR causes conformational changes in the intracellular loops of the receptor that in turn promote replacement of bound GDP for GTP on the Gα subunit [i.e., activated GPCRs exhibit guanine nucleotide exchange factor (GEF) activity (54)]. GTP binding changes the conformation of three flexible “switch” regions (switches I, II, and III) within Gα, allowing its dissociation from Gβγ (55). Gβγ and GTP-bound Gα subunits, once freed of one another, can each initiate signals by interacting with downstream effector proteins, including isoforms of adenylyl cyclase and phospholipase-C, as well as various ion channels.
Termination of signals generated by Gα•GTP and free Gβγ subunits is thought to rely on the intrinsic guanosine triphosphatase (GTPase) activity of Gα, an activity that can be augmented dramatically by members of the regulator of G protein signaling (RGS) family (56,,57). RGS proteins act as GTPase-activating proteins (GAPs), helping to return Gα to the GDP-bound state. Reassociation of Gβγ with GDP-bound Gα obscures critical effector contact sites within both partners, thereby terminating all effector interactions.
A NEW PARTNER FOR Gα
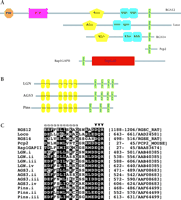
We first coined the term GoLoco motif (meaning Gαi/o–Loco interaction motif) following the identification of this conserved nineteen-residue sequence as a possible site of interaction with adenylyl cyclase–inhibitory Gi- and/or Go-subfamily Gα subunits (1). GoLoco motifs are found singly or repeated in tandem arrays within several diverse proteins (Figure 1⇓), including the Drosophila proteins Loco and Pins, and the mammalian proteins RGS12, RGS14, Purkinje cell protein-2 (Pcp2), Rap1GAP, LGN, and C10A [later termed activator of G protein signaling 3 (AGS3)]. Ponting also identified this same motif in LGN, RGS12, RGS14 and other open-reading frames through independent computational efforts directed toward reanalyzing the domain assignments for RGS12 and RGS14 (2).
GoLoco motifs in a diverse set of signaling regulatory proteins. Domain organization of single- (A) and multi-GoLoco (B) containing proteins as described by the Simple Modular Architecture Research Tool (SMART, http://smart.embl-heidelberg.de). C. Multiple sequence alignment of the nineteen-residue core GoLoco motif from indicated proteins. Conserved residues within the motif are boxed. Residues that form the N-terminal α-helix in the RGS14 GoLoco/Gαi1 crystal structure are denoted by “α” symbols. Arrowheads denote the highly conserved Asp/Glu-Gln-Arg triad. Peptide sequences were derived from GenBank and SWISS-PROT database records as denoted on right.
These in silico discoveries of the GoLoco motif protein family relied on previous studies in which several of these proteins were independently isolated in yeast two-hybrid assays utilizing Gα subunits as “bait.” Mochizuki and colleagues first identified a Gαi2-interacting protein they named LGN, based on the presence of N-terminal Leu-Gly-Asn repeats (3). Luo and Denker subsequently characterized Pcp2 as a Gαo-interacting protein in a similar fashion (4), and Granderath and colleagues identified Loco as a Drosophila Gαi-interacting protein after first isolating the loco locus in an enhancer-trap screen for genes critical to late-stage glial cell differentiation (5). Takesono and colleagues used a Saccharomyces cerevisiae-based functional screen, designed to identify mammalian activators of the GPCR-regulated yeast pheromone response, to isolate three distinct “activator of G protein signaling” (AGS) proteins (6). One of these, AGS3, exhibits high sequence similarity to LGN, particularly in the C-terminal region that contains four GoLoco motifs (alternatively described as “G protein regulatory (GPR)” motifs by Takesono and colleagues). [It is important to note that the other AGS proteins identified in this screen, AGS1 and AGS2, do not share GoLoco motifs or any other common structural feature, despite a previous confusing assertion in the literature (7). Rather, AGS1 is a Ras-related protein also known as Dexras1 (8), and AGS2 is a Gβγ-binding protein also known as Tctex1, a light chain component of the cytoplasmic motor protein dynein (6).]
Rules of the Dance
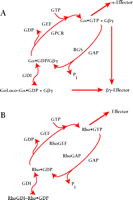
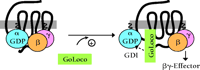
GoLoco-containing proteins interact preferentially with Gα subunits in the GDP-bound inactive state (6,9,,10) and do not bind with high affinity to active Gα (as mimicked by in vitro loading with the non-hydrolyzable GTP-analog GTPγ–S) nor to Gα subunits in the transition state for GTP hydrolysis (as mimicked by in vitro loading of Gα•GDP with the planar ion AlF4−) (11,,12). One possible outcome of such a specific interaction between GoLoco proteins and GDP-bound Gα subunits is the displacement of bound nucleotide, akin to receptor-catalyzed guanine nucleotide exchange factor (GEF) activity. Indeed, the original report of Luo and Denker ascribing such receptor-independent GEF activity to recombinant Pcp2 (4) led to the proposal that the GoLoco motif acts as a “potential guanine-nucleotide exchange factor” (1). However, using traditional [3H]-GDP release and [35S]-GTPγ–S binding assays, as well as a new fluorescence-based variation of the latter (Box 2), we and others have concluded that GoLoco protein binding to Gα•GDP slows the rate of GDP release, with an IC50 in the sub-micromolar range (9,10,13–,16). Thus, GoLoco motif–containing proteins function as GDIs for Gαi/o subunits (Box 2; Figure 2A⇓), and participate in the heterotrimeric G protein guanine nucleotide cycle in a fashion analogous to that of RhoGDI in the guanine nucleotide cycle of monomeric G proteins (Figure 2B⇓). Both the single GoLoco motif–containing proteins RGS12, RGS14, Pcp2, and Rap1GAP and the multi-GoLoco proteins AGS3 and LGN act as GDIs for Gαi subunits (9,10,13–,16). Although Pcp2 was initially reported as a GEF (4), wild-type Pcp2 does indeed have GDI activity in vitro (16).
Revised model of heterotrimeric G protein signaling. A. In comparison to the standard model shown in Box 1, this revised model introduces GoLoco proteins, as guanine nucleotide dissociation inhibitors (GDIs), into the traditional array of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) for Gα subunits (i.e., GPCR and RGS proteins, respectively). GoLoco binding to Gα•GDP results in the release of Gβγ independent of receptor-mediated GEF activity, thus allowing Gβγ to activate downstream effector pathways. B. By way of comparison, the current model of signaling by the small GTPase, Rho, involves GDI, GEF, and GAP activities in addition to effectors stimulated by Rho•GTP. However, the RhoGDI–Rho•GDP complex is thought to sequester Rho from membrane-delimited activation, and there is currently no evidence that this complex directly activates downstream signaling (48).
Ode to BODIPY.
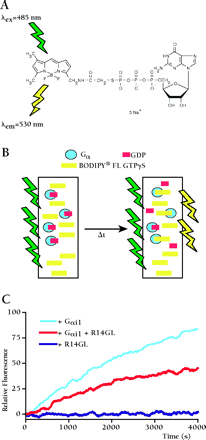
BODIPY® FL GTPγS is a fluorescent, non-hydrolyzable analog of GTP first employed by Neubig and colleagues (58) to replace radioactive [35S]-GTPγS filter-binding assays in monitoring the exchange of GDP for GTP by Gα subunits. We have used real-time BODIPY® FL GTPγS binding assays exclusively in our studies of guanine nucleotide dissociation inhibitor (GDI) activity exhibited by RGS12 and RGS14 GoLoco motifs (10,,17). As shown in panel A, the fluorescent BODIPY moiety is linked to GTPγS through the γ-phosphate. When in solution, the nucleoside folds back upon the BODIPY fluor, resulting in self-quenching and a low quantum yield. Upon binding to Gα subunits (panel B), the fluorescent moiety becomes exposed and exhibits an increased quantum yield that can be monitored in real time. For the experiment shown in Panel C, either 100 nM Gαi1, 100 nM Gαi1 + 100nM RGS14–GoLoco peptide (R14GL), or 100nM R14GL alone was added to a solution of 1 μM BODIPY® FL GTPγS in a 1 mL quartz cuvette. Each cuvette is excited at a wavelength of 485 nm and monitored for emission at 530 nm using a Perkin Elmer LS50B spectrofluorimeter. Using this system, we are able to perform four independent GTPγS binding experiments at one time. One can envision higher-throughput applications of this technique through automation. BODIPY® FL GTPγS is commercially available from Molecular Probes (http://www.probes.com).
So far, the GDI activity of GoLoco motif-containing proteins has been limited to subunits of the Gαi/o class. Some GoLoco proteins, including AGS3, RGS12, RGS14, and Pins, exhibit activity solely on the Gαi subunits Gαi1, Gαi2, and Gαi3, whereas Pcp2, Rap1GAP, and LGN are also able to bind to, and act as GDIs for, Gαo subunits (16,,17). The atomic–resolution structure of Gαi1•GDP bound to the GoLoco motif of RGS14 indicates that the selectivity of GoLoco proteins for particular Gα subunits is specified by the all-helical domain of the Gα subunit, and by a poorly conserved region directly C-terminal to the GoLoco motif (17). This finding provides a previously unrecognized function for the unique all-helical domain of heterotrimeric G protein α subunits, and also suggests that GoLoco motif–based GDIs might not be discovered for the Ras superfamily of monomeric GTPases, which lack an all-helical domain.
The Heart Of The Dance Routine
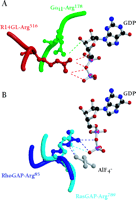
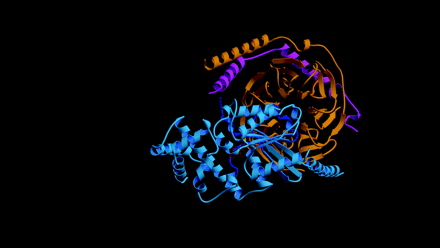
Knowing which of the specific residues within the GoLoco motif are critical for GDI activity might aid the rational design of small-molecule compounds that are able to regulate Gα nucleotide exchange. Lanier and colleagues have used alanine scanning and insertional mutagenesis to identify several residues within the GoLoco motif important for GDI activity (18). Unfortunately, the residues identified are distributed throughout a span of twenty residues and thus comprise an inordinately large surface area of interaction untenable for rational drug design. The recent solution of a crystal structure of Gαi1 bound to the GoLoco motif of RGS14 (PDB accession 1KJY, available at http://www.rcsb.org/pdb/index.html) suggests a more likely starting point for the design of small molecule GoLoco mimetics aimed at inhibiting nucleotide release (17). In the crystal structure, the GoLoco motif inserts the arginine residue of a highly conserved Asp/Glu-Gln-Arg triad (Figure 1C⇑) into the guanine nucleotide binding pocket; the guanidinium side-chain forms important contacts with the α and β phosphates of GDP (Figure 3A⇓). Mutation of this arginine to either alanine or leucine results in a nearly tenfold decrease in GDI activity with no corresponding change in binding affinity (17). However, mutation to phenylalanine, a bulky, hydrophobic residue that is sterically hindered from entering this binding pocket, destroys activity (6), presumably by grossly perturbing the binding affinity of the GoLoco–Gα interaction.
The arginine finger as a recurrent theme in G protein modulation. A. Arginine residues from both Gαi1 (green) and the RGS14-GoLoco peptide (“R14GL”; red) contact GDP in the crystal structure. B. The use of an arginine finger has also been observed in the crystal structures of RhoGAP and RasGAP with their respective GTPases. Atomic coordinates taken from GoLoco‴Gαi1 (1KJY), RhoGAP‴Rho (1TX4), and RasGAP‴Ras (1WQ1) were rendered using Molscript (49) and Raster3D (50).
An Old Dancestep Rechoreographed?
The use of an arginine side-chain to engage bound nucleotide is a recurrent theme in the modulation of G protein activity. Heterotrimeric G protein α subunits utilize an intrinsic arginine residue as one of the catalytic residues involved in GTP hydrolysis (e.g., Arg178 in Gαi1); mutation of this arginine results in a Gα subunit that is unable to hydrolyze GTP, thus rendering it constitutively active (19). After participating in the hydrolysis of the GTP γ-phosphate bond, this arginine makes hydrogen bonds with the α- and β-phosphates of the bound GDP (20). In the GoLoco–Gαi1•GDP crystal structure, the intrinsic arginine is displaced from the α- and β-phosphates so that the guanidinium group now hydrogen bonds with the ribose sugar of the GDP and participates in a salt bridge with Glu43 of the Gα subunit, a pairing also seen in the Gαi1•GDP–Gβγ heterotrimer (21). Thus, not one but two arginine residues engage GDP in the GoLoco-bound Gαi1 structure: one supplied in cis from the Gα subunit, and one supplied in trans from the GoLoco motif (Figure 3A⇑) (17).
Members of the Ras superfamily of small GTPases do not possess the intrinsic arginine residue common to their larger Gα subunit cousins, and thus have much slower intrinsic rates of GTP hydrolysis. GTPase activating proteins (GAPs) for the small GTPases supply this critical arginine “finger” in trans, facilitating the acceleration of GTP hydrolysis (22). Thus, the use of an arginine finger by the GoLoco motif to directly contact GDP is reminiscent of the arginine fingers of RasGAP and RhoGAP proteins, although the angle of approach (comparing Figure 3A⇑ to Figure 3B⇑) and functional consequences clearly differ.
Restricted Interdomain Movement For Gα?
The GDI effect of GoLoco proteins is not due entirely to the conserved arginine residue, because mutation of this important residue does not totally eliminate GDI activity (17). Another aspect of GDI activity may reside in the binding of the GoLoco protein across the nucleotide-binding pocket, forming a bridge between the all-helical and the Ras-like domains (17). Indeed, one current hypothesis as to how nucleotide exchange occurs in heterotrimeric G proteins is that the receptor, using Gβγ as a “lever,” induces Gα to open like a clamshell separating the Ras-like and all-helical domains and allowing the guanine nucleotide to leave the binding pocket (Figure 4A and 4B⇓⇓) (23). It therefore appears likely that the GoLoco “bridge” stabilizes the relative positions of the all-helical and Ras-like domains and thereby prevents clamshell breach and nucleotide egress (Figure 4C⇓).
Proposed mechanism for GoLoco-mediated GDI activity. A. In the Gαβγ heterotrimer, Gβγ subunits (orange and magenta, respectively) contact Gα (light blue) through the Ras-like domain and the associated switch regions (dark blue). GDP (brown) is not contacted by Gβγ, but is held tightly by the Gα subunit. B. GPCR activation is thought to change the orientation of the all-helical domain relative to the Ras-like domain (movement indicated by blue arrow), thus loosening Gα's grip on the nucleotide and allowing it to dissociate (brown arrow) (23). C. The GoLoco peptide acts as a Gα clamp by restricting movement of the two Gα domains relative to one another. The conserved nineteen-residue GoLoco motif (red) binds to the Ras-like domain and the switch regions while the C-terminal extension (green) stabilizes the all-helical domain. Atomic coordinates of Gαβγ taken from 1GOT.
A Solo Performance For Gβγ
While the GoLoco-motif restricts the release of GDP from Gα, the Gβγ heterodimer is freed from interacting with Gα•GDP. The solution of the Gαi1•GDP–GoLoco structure reveals a large-scale deformation of the conformationally flexible switch II within Gαi1•GDP upon GoLoco motif binding—a structural reorganization that precludes coincident Gβγ binding to GoLoco-complexed Gα•GDP (17). Such gross perturbation of a critical Gβγ contact site on Gα is consistent with previous reports that Gβγ- and GoLoco-binding to Gα•GDP are mutually exclusive (6,,16). Indeed, Gβγ is displaced from Gαi upon incubating Drosophila embryo lysates with either recombinant Pins protein or with a minimal C-terminal peptide (residues 601–638) encompassing the final GoLoco motif of Pins (Figure 1C⇑) (24).
These findings suggest that minimal GoLoco peptides could be used to prevent the reformation of G protein heterotrimers by maintaining free Gβγ heterodimers, which would permit signaling from Gβγ-specific effectors without simultaneously activating Gα•GTP–specific effectors (Figure 5⇓). Indeed, the ability of AGS3 to displace Gβγ from Gα•GDP is the most likely mechanism for explaining its identification in yeast as an activator of G protein signaling (6,8), given the central role of free Gβγ in yeast in triggering the downstream effector cascades. Moreover, the strict selectivity of certain GoLoco motifs, like those of RGS12 and RGS14, for Gαi subunits would not affect Gαo-containing heterotrimers. Such Gαi-selective inhibitors are lacking in the current pharmacological armamentarium used to study GPCR coupling; for example, pertussis toxin, commonly used to uncouple GPCR signaling, acts on both Gαi- and Gαo-containing heterotrimers (25). The use of GoLoco-peptides should help determine the relative contributions made by Gαi•GTP and Gβγ subunits to Gi-coupled GPCR signaling, as well as facilitate elucidation of the roles of heterotrimeric G proteins in newly appreciated mitotic processes underlying both asymmetric and symmetric cell divisions.
Perturbation of heterotrimeric G protein formation by GoLoco-motif peptides. Minimal peptides derived from GoLoco proteins could be used to disrupt the Gαβγ heterotrimer in the absence of GPCR activation. The binding of GoLoco peptides to Gα•GDP precludes Gβγ binding, thus allowing specific activation of Gβγ effectors without concomitant signaling from effectors modulated by Gα•GTP.
MOVING TO A NEW DANCE HALL
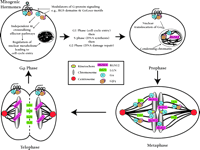
Until recently, the study of heterotrimeric G protein signaling in cell cycle control has concentrated on early mitogenic events centered in the transition from G0 to G1—events such as calcium mobilization, phospholipid metabolism, and protein kinase activation, which contribute to DNA synthesis and cellular proliferation caused by many GPCRs (26). New evidence suggests that heterotrimeric G protein signal transduction might participate in the underlying mechanics of mitotic cell division (Figure 6⇓).
The mammalian somatic cell cycle: Multiple levels of regulation by G protein signaling systems. Mitogenic hormones bind GPCRs to activate classical heterotrimeric G protein signaling pathways, leading to cell cycle entry and DNA synthesis. In addition, Gαi and the multidomain protein RGS12 are present on mitotic chromatin, and evidence suggests they may regulate cytokinesis and the exit from mitosis. Gαoβγ and the mammalian Pins analog LGN are present on the spindle microtubules and may directly regulate microtubule dynamics. LGN redistributes to the midbody structure separating daughter cells during late mitosis and may also participate in cytokinesis.
Starring Roles For Heterotrimeric G Proteins and Go Loco Proteins in Asymmetric Cell Division
Studies of the fruit fly Drosophila melanogaster and the nematode worm Caenorhabditis elegans suggest that heterotrimeric G protein signaling and, in particular, proteins containing GoLoco motifs, regulate asymmetric cell division (ACD). Usually, mitotic cell division results in equal distribution of the contents of the mother cell into its two daughters. In ACD, proteins and RNAs that participate in the determination of cell fate are distributed asymmetrically following division, resulting in distinctly different daughter cells (27).
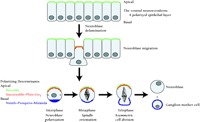
Drosophila neuroblast mitosis is a common example of ACD. Neuroblasts delaminate from the ventral neuroectoderm and adopt an apical-basal axis of polarity (Figure 7⇓). Subsequent asymmetric divisions produce large apical neuroblasts and smaller basal ganglion mother cells (GMCs). GMCs are committed to forming neurons or glia, whereas the apical neuroblasts can undergo repeated asymmetric divisions.
Asymmetric cell division in Drosophila neuroblasts. Neuroblasts delaminate from the ventral neuroectoderm. The cell polarity of this epithelium is maintained by apical protein complexes involving Bazooka, Inscuteable, Pins, and Gαi. Basal determinant molecules such as Numb, Prospero, and Miranda are also asymmetrically localized. The mitotic spindle is reoriented into an apical-basal axis, and asymmetric cell division occurs. The resultant apical neuroblasts can undergo further asymmetric divisions, whereas the smaller basal ganglion mother cells are committed to differentiating into neurons or glia.
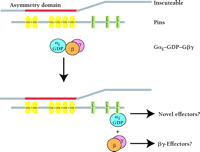
A protein integral to this program of neuroblast ACD is Inscuteable. Inscuteable is apically localized in neuroblasts and is responsible for the asymmetric distribution of several GMC determinants including the proteins Prospero and Numb (28). The molecular mechanisms by which Inscuteable regulates events has yet to be fully determined. To this end, deletion analysis of Inscuteable has defined a so-called “asymmetry domain” sufficient to mediate all the known functions of Inscuteable (29). Screens for proteins that interact with the asymmetry domain identified the unique protein Pins (Partner of Inscuteable) and a Gα subunit (Gαi/o) (30–,32). Pins contains seven tetratrico-peptide (TPR) repeats and three GoLoco motifs (Figure 8⇓). The location of Pins in neuroblasts is identical to that of Inscuteable, and Drosophila harboring pins null mutations are defective in ACD, a phenotype ascribed to changes in the location of Inscuteable (31).
Hypothetical mechanism of G protein activation during asymmetric cell division. Inscuteable uses its N-terminal “asymmetry domain” to bind to the tetratrico-peptide repeat (TPR) protein Pins. This interaction facilitates the polarized localization of both Pins and Gαi. Heterotrimeric Gαiβγ binds one or more of the GoLoco motifs present in Pins, causing subunit dissociation and the formation of Inscuteable–Pins–Gαi•GDP and free Gβγ complexes. Dissociated Gβγ and GoLoco-complexed Gαi could possibly regulate independent, as yet uncharacterized, effector pathways to mediate asymmetric cell division.
It Sometimes Takes Three To Tango
Gαi can form an asymmetrically distributed protein complex with Inscuteable and Pins, and requires both proteins for its polarized localization. Immunoprecipitation analyses have verified the existence of a stable Pins–Gαi•GDP complex (24). Furthermore, it appears that the repeated GoLoco-motifs in Pins cause Gβγ dissociation from Gαi and may thus effect an elegant receptor- and nucleotide-independent activation of Gβγ-dependent ACD. Consistent with this hypothesis, defects in ACD are observed in Drosophila cells that overexpress wild-type Gαi (which sequesters Gβγ), but ACD proceeds normally upon overexpression of a mutant, GTPase-deficient Gαi that cannot bind to Gβγ (24).
More Than One Venue For This Tango
The Pins–Gαi complex appears to regulate ACD in multiple situations. For example, Drosophila sensory organ precursor (SOP) cells also divide asymmetrically in an anterior-posterior fashion. This is an Inscuteable-independent process but exhibits a similar dependence on Pins-Gαi, as does neuroblast division (24). SOP cells are initially apico-basal in polarity and Pins is essential for switching the cells to an anterior-posterior asymmetry (33). In this situation, Pins is regulated by the proteins Frizzled and Discs-large. Interestingly, the Frizzled gene is thought to encode a functional GPCR (34), suggesting that receptor-mediated regulation of Gα subunits may, in some instances, be involved in generating polarity cues in ACD. The Drosophila RGS protein Loco, from which the GoLoco motif was first elucidated, is also essential for maintaining cell polarity. Disruption of loco expression in follicle cells leads to a ventralized embryo (35), further support for the idea that modulation of heterotrimeric G protein signaling may control cell polarization.
Heterotrimeric G Proteins Spin The Mitotic Spindle
For the successful completion of ACD, the mitotic spindle needs to be aligned with the axis of cell division. In the Drosophila neuroblast, spindle reorientation appears to occur during metaphase whereby the spindle rotates 90° to come into alignment with the apical-basal axis of division (36). Heterotrimeric G protein signaling appears to be involved in the process of spindle reorientation during ACD in both Drosophila and Caenorhabditis.
The very first cell division of C. elegans embryogenesis is asymmetric, producing a large anterior cell and a smaller posterior cell. C. elegans embryos deficient in both genomic- and maternally-encoded Gβ exhibit a randomized spindle orientation leading to abnormal tissue distribution and, ultimately, embryonic death (37). RNA-mediated interference (RNAi) studies suggest that Gαο, Gβ, and Gγ subunits all contribute to spindle position and orientation in the C. elegans embryo, with Gα regulating asymmetric placement and morphology of the first mitotic spindle, and Gβγ subunits controling centrosome migration (38). Both Gα and Gβ subunits are localized to mitotic spindles and are thus in potentially convenient locations to directly alter microtubule-dependent processes (37,,38). Two C. elegans proteins with GoLoco motifs (C38C10.4 and F22B7.13) might also participate in spindle positioning: concomitant inhibition of both GoLoco proteins results in a phenotype of essentially symmetric, rather than asymmetric, first cell division, a phenotype also seen upon concomitant interference of two Gαo subunits (GOA-1 and GPA-16) (38–,40).
SYMMETRY WITH MAMMALIAN SYSTEMS
Evidence of GoLoco protein involvement in Drosophila and Caenorhabditis ACD has recently been complemented by a study of a human Pins-related GoLoco protein, LGN, in symmetric mitosis. In mammalian cells, LGN moves from the nucleus to the midbody structure that separates daughter cells during late mitosis (41). LGN may be essential for the assembly and organization of the mitotic spindle: ectopic LGN overexpression, as well as silencing of LGN expression, disrupts spindle-pole organization and prevents normal chromosome segregation (42). Mitotic microtubule regulation by LGN appears to be directly mediated by the nuclear mitotic apparatus protein NuMA, a protein known to regulate microtubule function during mitosis (43). Direct interaction between LGN and NuMA is transacted through the first two TPR repeats of LGN (Figure 1B⇑), potentially leaving the C-terminal GoLoco-motif repeats free to interact with Gαο, which is also present on mitotic microtubules (Figure 6⇑).
Other G proteins of the Gαi/o class might also participate in mitotic regulation. In some mammalian cells (e.g. Swiss 3T3 fibroblasts), Gαi2 becomes nuclear-localized during prophase and stays bound to mitotic chromatin until cytokinesis has occurred (44,,45). Furthermore, Gαi2 is present in mitotic cells on kinetochores, multimolecular protein complexes responsible for connecting chromosomes with the spindle microtubules during mitosis (46). Treating these mammalian fibroblasts with pertussis toxin inhibits the function of Gαi, and blocks the cell cycle at the M/G1 transition, suggesting a functional role for Gαi in mitotic progression (46).
In addition, short-splice isoforms of RGS12 localize within the nucleus and specifically associate with chromatin during mitosis (Figure 6⇑) (47), similar to the localization found for Gαi2. Overexpression of specific RGS12 splice variants causes aberrant nuclear morphology and a multinucleated phenotype (47), implicating RGS12 in mitotic and cytokinetic processes, and correlating well with the evidence that Gαi regulates exit from mitosis.
UNANSWERED QUESTIONS
The involvement of GoLoco proteins, such as Pins, Loco, LGN, and RGS12, in the maintenance of cell polarity and orderly chromosomal and daughter-cell segregations raises many questions regarding the nature of heterotrimeric G protein signaling in these processes. What are the “effector(s)” for free Gβγ and GoLoco-bound Gα•GDP in these settings? Are they shared with plasma membrane-delimited GPCR signaling pathways or do they represent new nuclear activities? What role does repetition of the GoLoco motif play within LGN and Pins, given that the repeated motifs are seemingly too close together for the simultaneous binding of multiple Gα subunits? Is the association between GoLoco motifs and Gα subunits regulated by cell-cycle checkpoint machinery or other signaling inputs? Can GoLoco-motif peptides be used to perturb G protein heterotrimers to answer some of these questions? Our hope is that further exploration of this intimate dance between GoLoco-motif proteins and heterotrimeric G protein subunits will reveal the means by which cell polarity and cell division are controlled. New pharmacological agents may be valuable dividends of such studies, and may be able to curb uncontrolled division in cancerous states, or maintain the pluripotent nature of undifferentiated cells.
Acknowledgments
Work from the authors' laboratory is supported by NIH grants R01 GM62338 and P01 GM65533 to D.P.S, and F30 MH64319 to R.J.K. We would like to thank Michelle Kimple, John Sondek, and T. Kendall Harden for insightful comments and productive discussions. F.W. would also like to thank Michael Crouch for valuable discussions about G proteins in mitosis. D.P.S. is a Year 2000 Scholar of the EJLB Foundation (Montréal, Canada) and recipient of the Burroughs-Wellcome Fund New Investigator Award in the Basic Pharmacological Sciences.
- © American Society for Pharmacology and Experimental Theraputics 2002
References

David P. Siderovski, Ph.D.,(top left) is an Assistant Professor in the Department of Pharmacology at the Univeristy of North Carolina-Chapel Hill School of Medicine, and is a member of the UNC Neuroscience Center and the Lineberger Comprehensive Cancer Center. Randall J. Kimple, (top right) is an M.D.-Ph.D. student in David Siderovski's laboratory. Francis S. Willard, Ph.D., (right) is a post-doctoral fellow in David Siderovski's laboratory.