Emerging concepts from the recent literature
Neuropharmacological Insights from GABA Receptor Mutant Mice
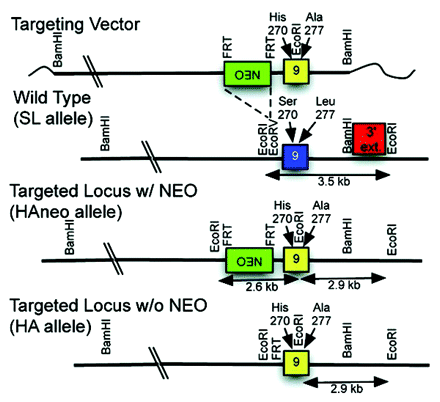
Despite ethanol’s long cultural history and its frequent use and abuse, an understanding of the molecular basis for its behavioral effects is lacking. Recent studies suggest that ethanol perturbs the function of the gamma-amino-butyric acid type A receptor (GABAA-R) ligand-gated ion channels, which are responsible for rapid inhibitory neuronal signals. A single S270H mutation in the α1 subunit of the GABAA-α1 receptor reduces anesthetic and alcohol sensitivity, but it has been difficult to relate this functional change to behavioral effects because the mutation also causes GABA hypersensitivity that confounds behavioral studies in the knock-in mouse. In their recent publication in JPET, Borghese and coworkers describe the development of a double GABAA-α1 mutant in which the second mutation normalizes GABA sensitivity. Electrophysiological measurements of double mutant transfected cell lines demonstrate normal chloride ion current sensitivity to GABA with attenuated potentiation sensitivity to ethanol and anesthetics. Development of a double mutant knock-in mouse is also described. In a companion paper by Werner et al., the same group describes behavioral studies of the double mutant knock-in mouse. The mutant and wild-type mice respond similarly to low doses of ethanol, but their responses are significantly perturbed at higher doses. The double knock-in mice are more sensitive to the anxiolytic effects of ethanol, but they recover better from ethanol-induced convulsions. The faster recovery rates from high dose ethanol- and etomidate-induced ataxia are consistent with hippocampal slice recordings, but at lower doses the more similar responses suggest involvement of a different GABAA-R subunit. Collectively, these two studies provide a clearer picture of which GABAA-R subunits might be responsible for the sedative and anxiolytic behaviors associated with volatile anesthetics and alcohol, which is the first step towards development of a better molecular understanding of ethanol’s behavioral neuropharmacology. [
] —C Beeson, Medical University of South Carolina
Shifting Eicosanoid Production: A New Strategy to Reduce Pain?
Non-steroidal anti-inflammatory drugs (NSAIDs) reduce inflammatory pain by inhibiting cyclooxygenases (COXs) but also have the potential for serious side effects. Epoxyeicosatrienoic acids (EETs), which are P450 metabolic products of arachidonic acid, have anti-inflammatory activity and are inactivated through hydrolysis by soluble epoxide hydrolase. In the September 12 issue of PNAS, Schmelzer et al. demonstrate that co-administration of NSAIDs with inhibitors of soluble epoxide hydrolase have additive effects in reducing lipopolysaccharide (LPS)-mediated sensitivity to noxious stimuli (hyperalgesia) in mice. The co-treatment increases the concentration of EETs in murine plasma and synergistically reduces the LPS-induced production of COX-2, PGD2, and PGE2. The drug combinations do not produce the type of imbalance in PGI2 and thromboxanes that has been implicated in cardiovascular toxicity after COX-2 inhibition. These experiments suggest that drug combinations that reduce inflammatory eicosanoids and increase anti-inflammatory eicosanoids might be useful to improve therapeutic efficacy while minimizing toxicity. [
] —MR Vasko, Indiana University School of Medicine
No NO Protection for the Broken Heart in Diabetes
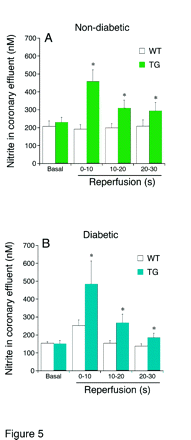
Cardiovascular complications are a leading cause of diabetic morbidity. Whereas much interest has focused upon the “vascular” portion, the consistent finding of impairment of cardiomyocyte function in diabetics and in animal models of the disease has led to the recognition of “diabetic cardiomyopathy” as a serious complication. In a recent publication in JPET, Pozo Navas et al. induced diabetes mellitus in a transgenic mouse model that constitutively overexpresses nitric oxide synthase in cardiomyocytes to explore the intersection between nitric oxide (NO) signaling and diabetes in heart cells. The authors show that the salubrious cardioprotective effects of NO in hearts challenged with ischemia-reperfusion are largely lost in the diabetic animals. Also, NO-induced increases in concentrations of cGMP are blunted in the diabetic animals. The detection of increased superoxide anion and/or peroxynitrite formation in the diabetic transgenic hearts suggests that the inactivation of NO by reactive oxygen species in the diabetic heart cell may underlie these observations. These studies add to our understanding of the dysfunction in heart that accompanies diabetes and raises the possibility that therapies based upon NO will be ineffective in diabetes unless reactive oxygen species generation is first controlled. [
] —GL Wright, Medical University of South Carolina
- © American Society for Pharmacology and Experimental Theraputics 2006



