STEPping into Position: A New Player in the NMDA Receptor Game
In the brain, protein phosphorylation and dephosphorylation control the efficacy of synaptic transmission and neuronal plasticity (1, 2). The importance of the concerted but competing actions of multiple protein kinases and protein phosphatases in neuronal cells is largely underscored by the necessity for bidirectional control of synaptic transmission. To maintain brain homeostasis and stability, it is essential that an increase in synaptic strength be followed by a resetting, or tuning down, of synaptic power. This resetting acts to guarantee a response from neurons when further stimulation is received and to prevent saturation, or unresponsiveness to stimulation. The notion that synaptic efficacy is modulated by selected sets of enzymes with opposing activities has become a prominent model in the field of learning and memory.
Protein kinases and phosphatases are intracellular components that convert electrical signals into biochemical outputs required for the processing and storage of information. According to a now classical model, protein kinases are activated during the induction and maintenance of synaptic strengthening such as long-term potentiation (LTP), whereas protein phosphatases are activated during synaptic weakening such as long-term depression (LTD) (3, 4). This view has been substantiated by numerous studies showing that kinase and phosphatase activities are changed after LTP or LTD, so that the phosphorylation and activity of the N-methyl-d-aspartate receptor (NMDAR), a glutamatergic receptor that mediates fast excitatory neurotransmission, is altered (5). However, a more complex picture is now emerging to suggest not only that kinases and phosphatases are simultaneously recruited, but that they can act at either common or distinct phosphorylation sites. Current knowledge about the types and the properties of the kinases and phosphatases involved and their mechanisms of action is, however, still incomplete.
Several families of protein kinases and phosphatases have been identified in the brain, and can be distinguished by the nature of the residues they modify (serine-threonine, or tyrosine). Both protein Ser-Thr–specific kinases (PSTKs) and phosphatases (PSTPs) are implicated in the regulation of neuronal functions. Numerous reports have demonstrated that the Ca2+-calmodulin–dependent kinase II (CaMKII), an abundant kinase in the brain, is critically involved in the control of synaptic strength (6). Its actions are in part regulated by a set of additional protein kinases and phosphatases from the same family, especially the 3’,5’-adenosine monophosphate (cAMP)–dependent protein kinase A (PKA) and the Ca2+-calmodulin–dependent protein phosphatase calcineurin (CN or PP2B). PKA and CN are thought to act concertedly to regulate CaMKII activity and thereby affect neuronal efficacy (6, 7).
But Ser-Thr phosphorylation is not the whole story. Tyr phosphorylation is now viewed as an additional mechanism that participates in the control of NMDAR activity. Several members of the Src family of protein Tyr kinases (PTKs) (i.e., Src, Fyn, and Abl) phosphorylate NMDAR and stimulate its activity as measured by increased ion flux (8). Protein Tyr phosphatases (PTPs) have also been implicated (9), and recently it was demonstrated that receptor dephosphorylation operates in a use-dependent manner in that it occurs only after the receptor is activated (10, 11). Here again, kinase activity seems to require a counterbalanced and time-regulated phosphatase activity. The identity of the protein phosphatases that are recruited for this function had remained unknown.
A recent article now describes the identification of a protein phosphatase that dephosphorylates the NMDAR (12). This protein is STEP61, a member of the STEP (striatal-enriched phosphatase) family of brain-enriched Tyr-specific and cytoplasmic phosphatases (13). Pelkey et al. demonstrate that STEP61 can associate with the NMDAR and, from this strategic position, directly oppose the action of Src (12). The localization of STEP61 to the cytoplasmic side of a membrane patch extracted from spinal cord neurons and containing NMDARs leads to a significant depression of NMDA channel activity. Further, microinjecton of STEP61 into these neurons reduces spontaneous activity of the NMDAR as measured by miniature excitatory postsynaptic currents (mEPSCs). Similarly, in CA1 pyramidal neurons from acute hippocampal slices, STEP61 prevents LTP after tetanic stimulation of the Schaffer collateral pathway, the major afferent pathway to CA1 neurons. Conversely, blocking endogenous STEP61 by using a specific antibody or a dominant-negative STEP61 mutant increases the NMDAR-dependent component of mEPSCs in both cultured spinal cord neurons and CA1 hippocampal neurons from brain slices, and this effect can be occluded by Src inhibition. This suggests that STEP and Src function antagonistically, acting to regulate each other’s effects on mEPSCs. Direct inhibition of STEP also enhances excitatory postsynaptic potentials (EPSPs)—an effect that can be occluded by LTP, which suggests that the inhibition of STEP61 may be part of the normal process of LTP formation. Finally, this enhancement also appears to require Ca2+ and to be specific to NMDARs without affecting AMPARs (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors). Overall, these results clearly demonstrate that STEP is a negative regulator of the NMDAR and acts by antagonizing Src in a tonic or continuous fashion. This antagonism of Src has a negative impact on LTP but its impact on LTD is unknown.
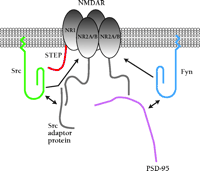
Although several PSTPs have already been reported to associate with specific subunits of the NMDAR (14, 15), the results of Pelkey et al. are the first to show that a specific PTP is also part of the NMDAR complex and that it specifically binds to the NR1 subunit of the NMDAR (12). These findings are interesting because they show that the actions of PTKs and PTPs are not limited to the regulation of ion channels (e.g., Na+, K+, or Ca2+ channels) but that they also contribute to synaptic plasticity through the control of neurotransmitter receptors. In turn, these results further complicate the already complex picture of the mechanisms of regulation of the NMDAR. Both tonic and activity-dependent (16) control are mediated through regulatory proteins that reside in a complex network of proteins. In this complex, regulation by kinases and phosphatases would seem to operate in a cross-reciprocal manner. STEP61, for instance, associates with and dephosphorylates Fyn (17), suggesting the interesting possibility that STEP may be a common regulator of Src and Fyn. Figure 1⇓ illustrates a potential arrangement of the complex.
How the actions of these multiple regulators are coordinated, and what specific role each has, will have to be determined. Furthermore, to fully understand the mechanisms of regulation of the NMDAR, it will be critical to determine what specific role each kinase–phosphatase pair plays in this process (e.g., whether acting on Tyr or Ser-Thr residues and which mechanisms determine their recruitment). The impact of the balanced phosphorylation-dephosphorylation is certainly not limited to the regulation of NMDAR functions but may also affect NMDAR trafficking and the coordination of protein–protein interactions in downstream signaling. Another feature of NMDARs possibly related to the concerted intervention of kinases and phosphatases is the differential regulation of synaptic versus extrasynaptic NMDARs. The distinct localization of these receptors has recently been shown to specify their roles (18). Tyr phosphorylation may be a mechanism involved in this specification. Finally, it will be important in the future to elucidate the role of STEP proteins in brain cells other than neurons: STEPs are expressed in reactive astrocytes after ischemia, suggesting that they participate in multiple normal and pathological brain processes.
A model for the complex of NMDAR-STEP-Src/Fyn. TheNR1 subunit interacts with STEP, whereas the NR2A subunit interacts indirectly with Src and Fyn through the Src adaptor protein and PSD-95 (adapted from 19). Arrows indicate the mechanisms of regulation between proteins. STEP, striatal-enriched phospatase.
- © American Society for Pharmacology and Experimental Theraputics 2002
References

Isabelle M. Mansuy, PhD, is an Assistant Professor of Neurobiology in the Department of Biology at the Swiss Federal Institute of Technology Zürich.